Abstract
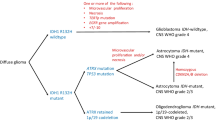
Medulloblastoma is heterogeneous, being characterized by molecular subgroups that demonstrate distinct gene expression profiles. Activation of the WNT or SHH signaling pathway characterizes two of these molecular subgroups, the former associated with low-risk disease and the latter potentially targeted by novel SHH pathway inhibitors. This manuscript reports the validation of a novel diagnostic immunohistochemical method to distinguish SHH, WNT, and non-SHH/WNT tumors and details their associations with clinical, pathological and cytogenetic variables. A cohort (n = 235) of medulloblastomas from patients aged 0.4–52 years was studied for expression of four immunohistochemical markers: GAB1, β-catenin, filamin A, and YAP1. Immunoreactivity (IR) for GAB1 characterizes only SHH tumors and nuclear IR for β-catenin only WNT tumors. IRs for filamin A and YAP1 identify SHH and WNT tumors. SHH, WNT, and non-SHH/WNT tumors contributed 31, 14, and 55% to the series. All desmoplastic/nodular (D/N) medulloblastomas were SHH tumors, while most WNT tumors (94%) had a classic phenotype. Monosomy 6 was strongly associated with WNT tumors, while PTCH1 loss occurred almost exclusively among SHH tumors. MYC or MYCN amplification and chromosome 17 imbalance occurred predominantly among non-SHH/WNT tumors. Among patients aged 3–16 years and entered onto the SIOP PNET3 trial, outcome was significantly better for children with WNT tumors, when compared to SHH or non-SHH/WNT tumors, which showed similar survival curves. However, high-risk factors (M+ disease, LC/A pathology, MYC amplification) significantly influenced survival in both SHH and non-SHH/WNT groups. We describe a robust method for detecting SHH, WNT, and non-SHH/WNT molecular subgroups in formalin-fixed medulloblastoma samples. In corroborating other studies that indicate the value of combining clinical, pathological, and molecular variables in therapeutic stratification schemes for medulloblastoma, we also provide the first outcome data based on a clinical trial cohort and novel data on how molecular subgroups are distributed across the range of disease.







Similar content being viewed by others

References
Al-Halabi H, Nantel A, Klekner A, Guiot MC, Albrecht S, Hauser P, Garami M, Bognar L, Kavan P, Gerges N, Shirinian M, Roberge D, Muanza T, Jabado N (2010) Preponderance of sonic hedgehog pathway activation characterizes adult medulloblastoma. Acta Neuropathol. doi:10.1007/s00401-010-0780-0 (on line, early release)
Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart CG, Lau CC, Olson JM, Gilbertson RJ, Gajjar A, Delattre O, Kool M, Ligon K, Meyerson M, Mesirov JP, Pomeroy SL (2010) Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. doi:10.1200/JCO.2010.28.5148 (on line, early release)
Clifford SC, Lusher ME, Lindsey JC, Langdon JA, Gilbertson RJ, Straughton D, Ellison DW (2006) Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell Cycle 5:2666–2670
Dahmen RP, Koch A, Denkhaus D, Tonn JC, Sorensen N, Berthold F, Behrens J, Birchmeier W, Wiestler OD, Pietsch T (2001) Deletions of AXIN1, a component of the WNT/wingless pathway, in sporadic medulloblastomas. Cancer Res 61:7039–7043
de Wit MC, Kros JM, Halley DJ, de Coo IF, Verdijk R, Jacobs BC, Mancini GM (2009) Filamin A mutation, a common cause for periventricular heterotopia, aneurysms and cardiac defects. J Neurol Neurosurg Psychiatry 80:426–428
Doggrell SA (2010) The hedgehog pathway inhibitor GDC-0449 shows potential in skin and other cancers. Expert Opin Investig Drugs 19:451–454
Dong J, Gailani MR, Pomeroy SL, Reardon D, Bale AE (2000) Identification of PATCHED mutations in medulloblastomas by direct sequencing. Hum Mutat 16:89–90
Ellison DW (2010) Childhood medulloblastoma: novel approaches to the classification of a heterogeneous disease. Acta Neuropathol 120:305–316
Ellison DW, Kocak M, Dalton J, Megahed H, Lusher ME, Ryan SL, Zhao W, Nicholson SL, Taylor RE, Bailey S, Clifford SC (2010) Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. doi:10.1200/JCO.2010.30.2810 (on line, early release)
Ellison DW, Onilude OE, Lindsey JC, Lusher ME, Weston CL, Taylor RE, Pearson AD, Clifford SC (2005) Beta-Catenin status predicts a favorable outcome in childhood medulloblastoma. J Clin Oncol 23:7951–7957
Evans DG, Farndon PA, Burnell LD, Gattamaneni HR, Birch JM (1991) The incidence of Gorlin syndrome in 173 consecutive cases of medulloblastoma. Br J Cancer 64:959–961
Fattet S, Haberler C, Legoix P, Varlet P, Lellouch-Tubiana A, Lair S, Manie E, Raquin MA, Bours D, Carpentier S, Barillot E, Grill J, Doz F, Puget S, Janoueix-Lerosey I, Delattre O (2009) Beta-catenin status in paediatric medulloblastomas: correlation of immunohistochemical expression with mutational status, genetic profiles, and clinical characteristics. J Pathol 218:86–94
Fernandez LA, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, Taylor MD, Kenney AM (2009) YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates sonic hedgehog-driven neural precursor proliferation. Genes Dev 23:2729–2741
Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, Fouladi M, Broniscer A, Krance R, Hale GA, Stewart CF, Dauser R, Sanford RA, Fuller C, Lau C, Boyett JM, Wallace D, Gilbertson RJ (2006) Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 7:813–820
Giangaspero F, Perilongo G, Fondelli MP, Brisigotti M, Carollo C, Burnelli R, Burger PC, Garre ML (1999) Medulloblastoma with extensive nodularity: a variant with favorable prognosis. J Neurosurg 91:971–977
Giangaspero F, Rigobello L, Badiali M, Loda M, Andreini L, Basso G, Zorzi F, Montaldi A (1992) Large-cell medulloblastomas. A distinct variant with highly aggressive behavior. Am J Surg Pathol 16:687–693
Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, Finkelstein D, Pounds S, Weiss A, Patay Z, Scoggins M, Ogg R, Pei Y, Yang ZJ, Brun S, Lee Y, Zindy F, Lindsey JC, Taketo MM, Boop FA, Sanford RA, Gajjar A, Clifford SC, Roussel MF, McKinnon PJ, Gutmann DH, Ellison DW, Wechsler-Reya R, Gilbertson RJ (2010) Subtypes of medulloblastoma have distinct developmental origins. Nature. doi:10.1038/nature09587 (on line, early release)
Gilbertson RJ, Ellison DW (2008) The origins of medulloblastoma subtypes. Annu Rev Pathol 3:341–365
Gillgrass A, Cardiff RD, Sharan N, Kannan S, Muller WJ (2003) Epidermal growth factor receptor-dependent activation of Gab1 is involved in ErbB-2-mediated mammary tumor progression. Oncogene 22:9151–9155
Giordana MT, Schiffer P, Lanotte M, Girardi P, Chio A (1999) Epidemiology of adult medulloblastoma. Int J Cancer 80:689–692
Goodrich LV, Milenkovic L, Higgins KM, Scott MP (1997) Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277:1109–1113
Huang H, Mahler-Araujo BM, Sankila A, Chimelli L, Yonekawa Y, Kleihues P, Ohgaki H (2000) APC mutations in sporadic medulloblastomas. Am J Pathol 156:433–437
Kimura H, Ng JM, Curran T (2008) Transient inhibition of the hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell 13:249–260
Kiyatkin A, Aksamitiene E, Markevich NI, Borisov NM, Hoek JB, Kholodenko BN (2006) Scaffolding protein Grb2-associated binder 1 sustains epidermal growth factor-induced mitogenic and survival signaling by multiple positive feedback loops. J Biol Chem 281:19925–19938
Koch A, Hrychyk A, Hartmann W, Waha A, Mikeska T, Schuller U, Sorensen N, Berthold F, Goodyer CG, Wiestler OD, Birchmeier W, Behrens J, Pietsch T (2007) Mutations of the Wnt antagonist AXIN2 (Conductin) result in TCF-dependent transcription in medulloblastomas. Int J Cancer 121:284–291
Koch A, Waha A, Tonn JC, Sorensen N, Berthold F, Wolter M, Reifenberger J, Hartmann W, Friedl W, Reifenberger G, Wiestler OD, Pietsch T (2001) Somatic mutations of WNT/wingless signaling pathway components in primitive neuroectodermal tumors. Int J Cancer 93:445–449
Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, Troost D, Meeteren NS, Caron HN, Cloos J, Mrsic A, Ylstra B, Grajkowska W, Hartmann W, Pietsch T, Ellison D, Clifford SC, Versteeg R (2008) Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One 3:e3088
Lamont JM, McManamy CS, Pearson AD, Clifford SC, Ellison DW (2004) Combined histopathological and molecular cytogenetic stratification of medulloblastoma patients. Clin Cancer Res 10:5482–5493
Louis DN (2007) WHO classification of tumours of the central nervous system. International Agency for Research on Cancer
McManamy CS, Pears J, Weston CL, Hanzely Z, Ironside JW, Taylor RE, Grundy RG, Clifford SC, Ellison DW (2007) Nodule formation and desmoplasia in medulloblastomas—defining the nodular/desmoplastic variant and its biological behavior. Brain Pathol 17:151–164
Nishida K, Yoshida Y, Itoh M, Fukada T, Ohtani T, Shirogane T, Atsumi T, Takahashi-Tezuka M, Ishihara K, Hibi M, Hirano T (1999) Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood 93:1809–1816
Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD (2010) Medulloblastoma comprises four distinct molecular variants. J Clin. doi:10.1200/JCO.2009.27.4324 (on line, early release)
Packer RJ (2010) Risk stratification of medulloblastoma: a paradigm for future childhood brain tumor management strategies. Curr Neurol Neurosci Rep. doi:10.1007/s11910-010-0168-5 (on line, early release)
Pfister S, Remke M, Benner A, Mendrzyk F, Toedt G, Felsberg J, Wittmann A, Devens F, Gerber NU, Joos S, Kulozik A, Reifenberger G, Rutkowski S, Wiestler OD, Radlwimmer B, Scheurlen W, Lichter P, Korshunov A (2009) Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol 27:1627–1636
Pietsch T, Waha A, Koch A, Kraus J, Albrecht S, Tonn J, Sorensen N, Berthold F, Henk B, Schmandt N, Wolf HK, von Deimling A, Wainwright B, Chenevix-Trench G, Wiestler OD, Wicking C (1997) Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res 57:2085–2088
Pizer BL, Clifford SC (2009) The potential impact of tumour biology on improved clinical practice for medulloblastoma: progress towards biologically driven clinical trials. Br J Neurosurg 23:364–375
Raffel C, Jenkins RB, Frederick L, Hebrink D, Alderete B, Fults DW, James CD (1997) Sporadic medulloblastomas contain PTCH mutations. Cancer Res 57:842–845
Reifenberger J, Wolter M, Weber RG, Megahed M, Ruzicka T, Lichter P, Reifenberger G (1998) Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res 58:1798–1803
Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, LoRusso PM, Von Hoff DD, de Sauvage FJ, Low JA (2009) Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med 361:1173–1178
Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, Graf N, Emser A, Pietsch T, Wolff JE, Kortmann RD, Kuehl J (2005) Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 352:978–986
Rutkowski S, von Hoff K, Emser A, Zwiener I, Pietsch T, Figarella-Branger D, Giangaspero F, Ellison DW, Garre ML, Biassoni V, Grundy RG, Finlay JL, Dhall G, Raquin MA, Grill J (2010) Survival and prognostic factors of early childhood medulloblastoma: an international meta-analysis. J Clin Oncol 28:4961–4968
Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, Agatep R, Chiappa S, Gao L, Lowrance A, Hao A, Goldstein AM, Stavrou T, Scherer SW, Dura WT, Wainwright B, Squire JA, Rutka JT, Hogg D (2002) Mutations in SUFU predispose to medulloblastoma. Nat Genet 31:306–310
Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J, Lucraft H, Gilbertson R, Tait DM, Walker DA, Pizer BL, Imeson J, Lashford LS (2003) Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 Study. J Clin Oncol 21:1581–1591
Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, Chintagumpala M, Adesina A, Ashley DM, Kellie SJ, Taylor MD, Curran T, Gajjar A, Gilbertson RJ (2006) Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol 24:1924–1931
Vorechovsky I, Tingby O, Hartman M, Stromberg B, Nister M, Collins VP, Toftgard R (1997) Somatic mutations in the human homologue of Drosophila patched in primitive neuroectodermal tumours. Oncogene 15:361–366
Wetmore C, Eberhart DE, Curran T (2001) Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res 61:513–516
Zhong Z, Yeow WS, Zou C, Wassell R, Wang C, Pestell RG, Quong JN, Quong AA (2010) Cyclin D1/cyclin-dependent kinase 4 interacts with filamin A and affects the migration and invasion potential of breast cancer cells. Cancer Res 70:2105–2114
Zhou AX, Hartwig JH, Akyurek LM (2010) Filamins in cell signaling, transcription and organ development. Trends Cell Biol 20:113–123
Zurawel RH, Allen C, Chiappa S, Cato W, Biegel J, Cogen P, de Sauvage F, Raffel C (2000) Analysis of PTCH/SMO/SHH pathway genes in medulloblastoma. Genes Chromosomes Cancer 27:44–51
Zurawel RH, Chiappa SA, Allen C, Raffel C (1998) Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Res 58:896–899
Acknowledgments
The authors gratefully acknowledge support from the American Lebanese Syrian Associated Charities. This study was conducted with appropriate ethics committee approval: St. Jude Children’s Research Hospital XPD07-107/IRB and Newcastle/North Tyneside REC 07/Q0905/71.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2011_800_MOESM1_ESM.docx
Supplementary Figure 1: Classic medulloblastoma. Most tumors demonstrate sheets of small densely packed round cells with a high nuclear:cytoplasmic ratio (a), although 14% of classic medulloblastomas in this series contained elongated cells arranged in a vague fascicular pattern (b). A small proportion (7%) of classic tumors showed nodules of differentiated neurocytic cells (c), without surrounding desmoplasia (d; reticulin stain). Other manifestations of focal neuronal differentiation included dense aggregates of tiny neurocytes (e) or irregular regions of neurocytes and ganglion cells against a neuropil-like matrix (f). Non-desmoplastic nodular regions (g) and dense neurocytic clusters (h) express neuronal proteins, such as synaptophysin (illustrated). All foci of neuronal differentiation had a low growth fraction, as estimated by Ki-67 immunolabeling in illustrated for this neurocytic cluster (i). Foci of astrocytic differentiation are exceptional (j). (DOCX 4811 kb)
401_2011_800_MOESM2_ESM.docx
Supplementary Figure 2: Desmoplastic medulloblastoma. The archetypal desmoplastic/nodular medulloblastoma contains scattered nodules of neurocytes against a neuropil-like matrix (a). Nodules are separated by reticulin-rich desmoplastic regions (b; reticulin stain). The MBEN is characterized by large irregular nodules that dominate the tumor’s architecture (c). In the MBEN, strikingly monomorphic neurocytes may form ribbons in elongated nodules and are often separated from a nodule’s periphery by an anuclear zone (d). Strong expression of NEU-N characterizes intranodular neurocytes (e), while there is a marked disparity in growth fraction between nodular and internodular regions (f; Ki-67 immunoreactivity). Paucinodular medulloblastomas show widespread desmoplasia, among which small nodules can be detected (g). The nodules do not have the low cell density of the MBEN, and a neurocytic morphology is subtle, but expression of neuronal proteins can be demonstrated (h; NEU-N immunoreactivity). Intranodular anaplasia may be a feature of some D/N medulloblastomas (i), as may invasion of nodules by embryonal cells (j). (DOCX 3133 kb)
401_2011_800_MOESM3_ESM.docx
Supplementary Figure 3: Large cell/anaplastic medulloblastoma. Anaplastic medulloblastomas show marked cytological pleomorphism, with molding of polyhedral nuclei against one another, cell wrapping, and high mitotic and apoptotic counts (a, b, c). Despite no obvious morphological differentiation, anaplastic tumors usually express neuronal markers, including neurofilament proteins, which are rarely expressed in classic tumors (d; NFP immunoreactivity). The large cell phenotype is different from anaplastic morphology and demonstrates groups of uniform large cells with prominent nucleoli (e). A few tumors from infants demonstrated sheets of uniform round cells with one or two prominent nucleoli (f). (DOCX 2130 kb)
401_2011_800_MOESM4_ESM.docx
Supplementary Figure 4: Idiosyncratic perivascular niche phenotype. A perivascular arrangement of anaplastic embryonal cells gives way to a more differentiated phenotype, with neurocytic cells against a neuropil-like matrix, away from blood vessels (a, b). (DOCX 748 kb)
401_2011_800_MOESM5_ESM.doc
Supplementary Figure 5: Interphase FISH. Monosomy 6 (a). PTCH1 (green signal) loss (b). Isodicentric 17q (c). MYC amplification (d). (DOC 211 kb)
Rights and permissions
About this article
Cite this article
Ellison, D.W., Dalton, J., Kocak, M. et al. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol 121, 381–396 (2011). https://doi.org/10.1007/s00401-011-0800-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0800-8