Abstract
Introduction
Intravesical therapy has been an important aspect of the management of non-muscle invasive bladder cancer (NMIBC) for 40 years. Bacillus Calmette–Guerin (BCG) is considered standard of care for intermediate and high-grade non-invasive disease, yet understanding the nuances of subsequent intravesical therapy is important for any provider managing bladder cancer. Herein, we review the literature and describe optimal use of intravesical therapies for NMIBC.
Methods
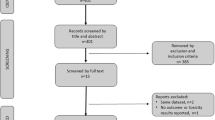
A comprehensive search of the medical literature was performed and highlighted in this review of intravesical therapy for NMIBC.
Results
Post-resection intravesical Mitomycin C therapy for low-risk disease remains an important component of care, and gemcitabine now has level-one evidence demonstrating efficacy in this setting but is not yet a guideline recommendation. BCG intravesical therapy remains the most effective therapy preventing recurrence and progression of intermediate and high-risk NMIBC. Adequately characterizing BCG-failure is critical in determining the next step in management which includes radical cystectomy, additional intravesical immunotherapy, chemotherapy with intravesical gemcitabine ± docetaxel and clinical trials.
Conclusions
Intravesical therapy remains the mainstay of treatment for NMIBC and bladder preservation. Intravesical induction BCG followed by maintenance therapy remains standard of care for intermediate and high-risk patients. Detailing the timing and characteristics of recurrence after intravesical therapy is crucial in determining subsequent treatment recommendations. Current clinical trials focus on systemic immunotherapy and enhancing the intravesical immune response by augmenting the delivery mechanism.

Adapted from Steinberg et al. [73]
Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics, 2017. CA Cancer J Clin 67(1):7–30
Abdollah F, Gandaglia G, Thuret R et al (2013) Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol 37(3):219–225
Nielsen ME, Smith AB, Meyer AM et al (2014) Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the United States: 1988 to 2006. Cancer 120(1):86–95
Babjuk M, Bohle A, Burger M et al (2017) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol 71(3):447–461
Chang SS, Boorjian SA, Chou R et al (2016) Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol 196(4):1021–1029
Kamat AM, Flaig TW, Grossman HB et al (2015) Expert consensus document: consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nat Rev Urol. 12(4):225–235
Brausi M, Collette L, Kurth K et al (2002) Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol 41(5):523–531
Herr HW (1999) The value of a second transurethral resection in evaluating patients with bladder tumors. J Urol 162(1):74–76
Divrik RT, Yildirim U, Zorlu F, Ozen H (2006) The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: a prospective, randomized clinical trial. J Urol 175(5):1641–1644
Sfakianos JP, Kim PH, Hakimi AA, Herr HW (2014) The effect of restaging transurethral resection on recurrence and progression rates in patients with nonmuscle invasive bladder cancer treated with intravesical bacillus Calmette–Guerin. J Urol 191(2):341–345
Morales A, Eidinger D, Bruce AW (1976) Intracavitary bacillus Calmette–Guerin in the treatment of superficial bladder tumors. J Urol 116(2):180–183
Kamat AM, Porten S (2014) Myths and mysteries surrounding bacillus Calmette–Guerin therapy for bladder cancer. Eur Urol 65(2):267–269
Witjes JA, Palou J, Soloway M et al (2013) Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette–Guerin (BCG): results of an international individual patient data survey (IPDS). BJU Int. 112(6):742–750
Messing EM, Tangen CM, Lerner SP et al (2018) Effect of intravesical instillation of gemcitabine vs saline immediately following resection of suspected low-grade non-muscle-invasive bladder cancer on tumor recurrence: SWOG S0337 randomized clinical trial. JAMA 319(18):1880–1888
Shore ND, Boorjian SA, Canter DJ et al (2017) Intravesical rAd-IFNalpha/Syn3 for patients with high-grade, bacillus Calmette–Guerin-refractory or relapsed non-muscle-invasive bladder cancer: a phase II randomized study. J Clin Oncol 35(30):3410–3416
Brausi M, Witjes JA, Lamm D et al (2011) A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol 186(6):2158–2167
Cambier S, Sylvester RJ, Collette L et al (2016) EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus Calmette–Guerin. Eur Urol 69(1):60–69
Gontero P, Sylvester R, Pisano F et al (2015) Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with bacillus Calmette–Guerin: results of a retrospective multicenter study of 2451 patients. Eur Urol 67(1):74–82
Sylvester RJ, van der Meijden AP, Oosterlinck W et al (2006) Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49(3):466–477 (discussion 467–475)
Kamat AM, Sylvester RJ, Bohle A et al (2016) Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the International Bladder Cancer Group. J Clin Oncol 34(16):1935–1944
Kamat AM, Witjes JA, Brausi M et al (2014) Defining and treating the spectrum of intermediate risk nonmuscle invasive bladder cancer. J Urol 192(2):305–315
Sylvester RJ, Oosterlinck W, Holmang S et al (2016) Systematic review and individual patient data meta-analysis of randomized trials comparing a single immediate instillation of chemotherapy after transurethral resection with transurethral resection alone in patients with stage pTa–pT1 urothelial carcinoma of the bladder: which patients benefit from the instillation? Eur Urol 69(2):231–244
Bosschieter J, Nieuwenhuijzen JA, van Ginkel T et al (2018) Value of an immediate intravesical instillation of mitomycin C in patients with non-muscle-invasive bladder cancer: a prospective multicentre randomised study in 2243 patients. Eur Urol 73(2):226–232
Cookson MS, Chang SS, Oefelein MG, Gallagher JR, Schwartz B, Heap K (2012) National practice patterns for immediate postoperative instillation of chemotherapy in nonmuscle invasive bladder cancer. J Urol 187(5):1571–1576
Filson CP, Montgomery JS, Dailey SM et al (2014) Complications associated with single-dose, perioperative mitomycin-C for patients undergoing bladder tumor resection. Urol Oncol 32(1):40 e41–40 e48
Barocas DA, Liu A, Burks FN et al (2013) Practice based collaboration to improve the use of immediate intravesical therapy after resection of nonmuscle invasive bladder cancer. J Urol 190(6):2011–2016
Davies BJ, Hwang TJ, Kesselheim AS (2017) Ensuring access to injectable generic drugs—the case of intravesical BCG for bladder cancer. N Engl J Med 376(15):1401–1403
Bohle A, Jocham D, Bock PR (2003) Intravesical bacillus Calmette–Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol 169(1):90–95
Malmstrom PU, Sylvester RJ, Crawford DE et al (2009) An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette–Guerin for non-muscle-invasive bladder cancer. Eur Urol 56(2):247–256
Sylvester RJ, Brausi MA, Kirkels WJ et al (2010) Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette–Guerin, and bacillus Calmette–Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol 57(5):766–773
Oddens J, Brausi M, Sylvester R et al (2013) Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette–Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol 63(3):462–472
Lamm DL, Blumenstein BA, Crissman JD et al (2000) Maintenance bacillus Calmette–Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 163(4):1124–1129
Sylvester RJ, van der Meijden AP, Witjes JA, Kurth K (2005) Bacillus calmette–Guerin versus chemotherapy for the intravesical treatment of patients with carcinoma in situ of the bladder: a meta-analysis of the published results of randomized clinical trials. J Urol 174(1):86–91 (discussion 82–91)
Akaza H, Hinotsu S, Aso Y, Kakizoe T, Koiso K (1995) Bacillus Calmette-Guerin treatment of existing papillary bladder cancer and carcinoma in situ of the bladder. Four-year results. The Bladder Cancer BCG Study Group. Cancer 75(2):552–559
Badalament RA, Herr HW, Wong GY et al (1987) A prospective randomized trial of maintenance versus nonmaintenance intravesical bacillus Calmette–Guerin therapy of superficial bladder cancer. J Clin Oncol 5(3):441–449
Koga H, Ozono S, Tsushima T et al (2010) Maintenance intravesical bacillus Calmette–Guerin instillation for Ta, T1 cancer and carcinoma in situ of the bladder: randomized controlled trial by the BCG Tokyo Strain Study Group. Int J Urol 17(9):759–766
Palou J, Laguna P, Millan-Rodriguez F, Hall RR, Salvador-Bayarri J, Vicente-Rodriguez J (2001) Control group and maintenance treatment with bacillus Calmette–Guerin for carcinoma in situ and/or high grade bladder tumors. J Urol 165(5):1488–1491
Witjes JA, Dalbagni G, Karnes RJ et al (2016) The efficacy of BCG TICE and BCG Connaught in a cohort of 2,099 patients with T1G3 non-muscle-invasive bladder cancer. Urol Oncol 34(11):484 e419–484 e425
Duchek M, Johansson R, Jahnson S et al (2010) Bacillus Calmette-Guerin is superior to a combination of epirubicin and interferon-alpha2b in the intravesical treatment of patients with stage T1 urinary bladder cancer. A prospective, randomized, Nordic study. Eur Urol 57(1):25–31
Shepherd AR, Shepherd E, Brook NR (2017) Intravesical bacillus Calmette-Guerin with interferon-alpha versus intravesical bacillus Calmette-Guerin for treating non-muscle-invasive bladder cancer. Cochrane Database Syst Rev 3:CD012112
de Reijke TM, Kurth KH, Sylvester RJ et al (2005) Bacillus Calmette–Guerin versus epirubicin for primary, secondary or concurrent carcinoma in situ of the bladder: results of a European Organization for the Research and Treatment of Cancer–Genito-Urinary Group Phase III Trial (30906). J Urol 173(2):405–409
Herr HW, Milan TN, Dalbagni G (2015) BCG-refractory vs. BCG-relapsing non-muscle-invasive bladder cancer: a prospective cohort outcomes study. Urol Oncol 33(3):108 e101–108 e104
Lerner SP, Dinney C, Kamat A et al (2015) Clarification of bladder cancer disease states following treatment of patients with intravesical BCG. Bladder Cancer 1(1):29–30
Steinberg RL, Thomas LJ, Mott SL, O’Donnell MA (2016) Bacillus Calmette–Guerin (BCG) treatment failures with non-muscle invasive bladder cancer: a data-driven definition for BCG unresponsive disease. Bladder Cancer 2(2):215–224
Kamat AM, Lerner S, Black P et al (2017) Once BCG unresponsive, always BCG unresponsive: an open letter to the FDA to enhance recruitment into clinical trials in bladder cancer. Bladder Cancer 3(3):145–146
Li R, Tabayoyong WB, Guo CC et al (2018) Prognostic implication of the United States food and drug administration-defined BCG-unresponsive disease. Eur Urol. https://doi.org/10.1016/j.eururo.2018.09.028
Malmstrom PU, Wijkstrom H, Lundholm C, Wester K, Busch C, Norlen BJ (1999) 5-year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guerin in patients with superficial bladder carcinoma. Swedish-Norwegian Bladder Cancer Study Group. J Urol 161(4):1124–1127
Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M (2000) Efficacy and safety of valrubicin for the treatment of bacillus Calmette–Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol 163(3):761–767
Dinney CP, Greenberg RE, Steinberg GD (2013) Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette–Guerin. Urol Oncol 31(8):1635–1642
O’Donnell MA, Boehle A (2006) Treatment options for BCG failures. World J Urol 24(5):481–487
Catalona WJ, Hudson MA, Gillen DP, Andriole GL, Ratliff TL (1987) Risks and benefits of repeated courses of intravesical bacillus Calmette–Guerin therapy for superficial bladder cancer. J Urol 137(2):220–224
Belldegrun AS, Franklin JR, O’Donnell MA et al (1998) Superficial bladder cancer: the role of interferon-alpha. J Urol 159(6):1793–1801
Martini T, Wezel F, Lobig N, Mitterberger MJ, Colleselli D (2017) Systematic review on conservative treatment options in non-muscle-invasive bladder cancer patients refractory to bacillus Calmette–Guerin instillation therapy. Aktuelle Urol 48(4):314–328
Yates DR, Brausi MA, Catto JW et al (2012) Treatment options available for bacillus Calmette–Guerin failure in non-muscle-invasive bladder cancer. Eur Urol 62(6):1088–1096
Nativ O, Witjes JA, Hendricksen K et al (2009) Combined thermo-chemotherapy for recurrent bladder cancer after bacillus Calmette–Guerin. J Urol 182(4):1313–1317
Soria F, Milla P, Fiorito C et al (2016) Efficacy and safety of a new device for intravesical thermochemotherapy in non-grade 3 BCG recurrent NMIBC: a phase I–II study. World J Urol 34(2):189–195
Lammers RJ, Witjes JA, Inman BA et al (2011) The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. Eur Urol 60(1):81–93
Joudi FN, Smith BJ, O’Donnell MA, National BCGIPIG (2006) Final results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urol Oncol 24(4):344–348
Lamm D, Brausi M, O’Donnell MA, Witjes JA (2014) Interferon alfa in the treatment paradigm for non-muscle-invasive bladder cancer. Urol Oncol 32(1):35 e21–35 e30
Nepple KG, Lightfoot AJ, Rosevear HM, O’Donnell MA, Lamm DL, Bladder Cancer Genitourinary Oncology Study G (2010) Bacillus Calmette-Guerin with or without interferon alpha-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. J Urol 184(5):1915–1919
Xiao Z, Hanel E, Mak A, Moore RB (2011) Antitumor efficacy of intravesical BCG, gemcitabine, interferon-alpha and interleukin-2 as mono- or combination-therapy for bladder cancer in an orthotopic tumor model. Clin Med Insights Oncol. 5:315–323
O’Donnell MA, Luo Y, Chen X, Szilvasi A, Hunter SE, Clinton SK (1999) Role of IL-12 in the induction and potentiation of IFN-gamma in response to bacillus Calmette–Guerin. J Immunol. 163(8):4246–4252
Addeo R, Caraglia M, Bellini S et al (2010) Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: evaluation of efficacy and tolerance. J Clin Oncol 28(4):543–548
Di Lorenzo G, Perdona S, Damiano R et al (2010) Gemcitabine versus bacille Calmette–Guerin after initial bacille Calmette–Guerin failure in non-muscle-invasive bladder cancer: a multicenter prospective randomized trial. Cancer 116(8):1893–1900
Skinner EC, Goldman B, Sakr WA et al (2013) SWOG S0353: phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette–Guerin. J Urol 190(4):1200–1204
Laudano MA, Barlow LJ, Murphy AM et al (2010) Long-term clinical outcomes of a phase I trial of intravesical docetaxel in the management of non-muscle-invasive bladder cancer refractory to standard intravesical therapy. Urology 75(1):134–137
Barlow LJ, McKiernan JM, Benson MC (2013) Long-term survival outcomes with intravesical docetaxel for recurrent nonmuscle invasive bladder cancer after previous bacillus Calmette–Guerin therapy. J Urol 189(3):834–839
Breyer BN, Whitson JM, Carroll PR, Konety BR (2010) Sequential intravesical gemcitabine and mitomycin C chemotherapy regimen in patients with non-muscle invasive bladder cancer. Urol Oncol 28(5):510–514
Lightfoot AJ, Breyer BN, Rosevear HM, Erickson BA, Konety BR, O’Donnell MA (2014) Multi-institutional analysis of sequential intravesical gemcitabine and mitomycin C chemotherapy for non-muscle invasive bladder cancer. Urol Oncol 32(1):35 e15–35 e39
Cockerill PA, Knoedler JJ, Frank I, Tarrell R, Karnes RJ (2016) Intravesical gemcitabine in combination with mitomycin C as salvage treatment in recurrent non-muscle-invasive bladder cancer. BJU Int 117(3):456–462
Velaer KN, Steinberg RL, Thomas LJ, O’Donnell MA, Nepple KG (2016) Experience with sequential intravesical gemcitabine and docetaxel as salvage therapy for non-muscle invasive bladder cancer. Curr Urol Rep 17(5):38
Milbar N, Kates M, Chappidi MR et al (2017) Oncological outcomes of sequential intravesical gemcitabine and docetaxel in patients with non-muscle invasive bladder cancer. Bladder Cancer 3(4):293–303
Steinberg RL, Thomas LJ, Nepple KG (2016) Intravesical and alternative bladder-preservation therapies in the management of non-muscle-invasive bladder cancer unresponsive to bacillus Calmette–Guerin. Urol Oncol 34(6):279–289
Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Algaba F, Vicente-Rodriguez J (2000) Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol 164(3 Pt 1):680–684
Herr HW, Sogani PC (2001) Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol 166(4):1296–1299
Inman BA, Sebo TJ, Frigola X et al (2007) PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer 109(8):1499–1505
Packiam VT, Lamm DL, Barocas DA et al. (2017) An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: interim results. Urol Oncol 36(10):440–447. https://doi.org/10.1016/j.urolonc.2017.07.005
Funding
No funding was required for this work.
Author information
Authors and Affiliations
Contributions
CP: protocol/project development, data collection/management, analysis, manuscript writing, supervision. JC: data collection/management, analysis, manuscript writing, critical revisions. MA: analysis, manuscript editing. AK: critical revisions, supervision. SG: protocol/project development, data collection/management, analysis, manuscript editing, critical revisions, supervision. PS: protocol/project development, project management, critical revisions, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest or relevant disclosures to this work.
Informed consent
No patient data was included in this report and informed consent was not required.
Rights and permissions
About this article
Cite this article
Peyton, C.C., Chipollini, J., Azizi, M. et al. Updates on the use of intravesical therapies for non-muscle invasive bladder cancer: how, when and what. World J Urol 37, 2017–2029 (2019). https://doi.org/10.1007/s00345-018-2591-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2591-1