Abstract
Objectives
To evaluate the correlation between grade of hepatic neuroendocrine tumours (NETs) according to the 2010 World Health Organization (WHO) classification and the apparent diffusion coefficient (ADC) and to assess whether ADC value can predict overall survival (OS) after diagnosis of hepatic NETs.
Methods
The study included 63 patients who underwent magnetic resonance (MR) imaging with diffusion-weighted images for the evaluation of hepatic NETs. The correlation between qualitative and quantitative MR imaging findings, including ADC values, and WHO classifications was assessed. The association between ADC value and OS was analyzed.
Results
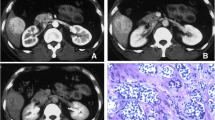
The ADC values and WHO classification of hepatic NETs were moderately negatively correlated in a statistically significant manner (ρ = −0.57, p < 0.001). The OS rates were significantly different according to the ADC value (low ADC vs. high ADC, p = 0.006) as well as WHO classifications (G1+ G2 vs. G3, p = 0.038). However, multivariate analysis revealed that the only independent predictor for OS was a low ADC value (hazard ratio: 3.37, p = 0.010).
Conclusion
There was a significant correlation between the ADC value of hepatic NETs and the WHO tumour grade. Additionally, the ADC value of a hepatic NET might be more accurate than the current WHO tumour grade for predicting OS.
Key points
• ADC values of hepatic NET and WHO tumour grade were negatively correlated.
• Lower ADC values of hepatic NET were significantly correlated with worse OS.
• ADC value might be more accurate than WHO grade for predicting OS.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CI:
-
Confidence interval
- DWI:
-
Diffusion-weighted image
- HR:
-
Hazard ratio
- MR:
-
Magnetic resonance
- NET:
-
Neuroendocrine tumour
- OS:
-
Overall survival
- PACS:
-
Picture archiving and communication system
- ROI:
-
Region-of-interest
- ROC:
-
Receiver operating characteristic
- WHO:
-
World Health Organization
References
Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S (2010) The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 39:707–712
Saxena A, Chua TC, Perera M, Chu F, Morris DL (2012) Surgical resection of hepatic metastases from neuroendocrine neoplasms: a systematic review. Surg Oncol 21:e131–e141
Cho CS, Labow DM, Tang L et al (2008) Histologic grade is correlated with outcome after resection of hepatic neuroendocrine neoplasms. Cancer 113:126–134
Saxena A, Chua TC, Sarkar A et al (2011) Progression and survival results after radical hepatic metastasectomy of indolent advanced neuroendocrine neoplasms (NENs) supports an aggressive surgical approach. Surgery 149:209–220
Anlauf M (2011) Neuroendocrine neoplasms of the gastroenteropancreatic system: pathology and classification. Horm Metab Res 43:825–831
Pasaoglu E, Dursun N, Ozyalvacli G, Hacihasanoglu E, Behzatoglu K, Calay O (2015) Comparison of World Health Organization 2000/2004 and World Health Organization 2010 classifications for gastrointestinal and pancreatic neuroendocrine tumors. Ann Diagn Pathol 19:81–87
Kloppel G (2011) Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 18(Suppl 1):S1–16
Padhani AR, Liu G, Koh DM et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Kim M, Kang TW, Kim YK et al (2016) Pancreatic neuroendocrine tumour: Correlation of apparent diffusion coefficient or WHO classification with recurrence-free survival. Eur J Radiol 85:680–687
Jang KM, Kim SH, Lee SJ, Choi D (2014) The value of gadoxetic acid-enhanced and diffusion-weighted MRI for prediction of grading of pancreatic neuroendocrine tumors. Acta Radiol 55:140–148
Kim JH, Eun HW, Kim YJ, Han JK, Choi BI (2013) Staging accuracy of MR for pancreatic neuroendocrine tumor and imaging findings according to the tumor grade. Abdom Imaging 38:1106–1114
Kang TW, Kim SH, Jang KM et al (2015) Gastrointestinal stromal tumours: correlation of modified NIH risk stratification with diffusion-weighted MR imaging as an imaging biomarker. Eur J Radiol 84:33–40
Attenberger UI, Pilz LR, Morelli JN et al (2014) Multi-parametric MRI of rectal cancer - do quantitative functional MR measurements correlate with radiologic and pathologic tumor stages? Eur J Radiol 83:1036–1043
Akashi M, Nakahusa Y, Yakabe T et al (2014) Assessment of aggressiveness of rectal cancer using 3-T MRI: correlation between the apparent diffusion coefficient as a potential imaging biomarker and histologic prognostic factors. Acta Radiol 55:524–531
ZH L, CH H, Qian WX, Cao WH (2016) Preoperative diffusion-weighted imaging value of rectal cancer: preoperative T staging and correlations with histological T stage. Clin Imaging 40:563–568
Shankar S, Kalra N, Bhatia A et al (2016) Role of Diffusion Weighted Imaging (DWI) for Hepatocellular Carcinoma (HCC) Detection and its Grading on 3T MRI: A Prospective Study. J Clin Exp Hepatol 6:303–310
Wang LX, Liu K, Lin GW, Jiang T (2015) Primary hepatic neuroendocrine tumors: comparing CT and MRI features with pathology. Cancer Imaging 15:13
Besa C, Ward S, Cui Y, Jajamovich G, Kim M, Taouli B (2016) Neuroendocrine liver metastases: Value of apparent diffusion coefficient and enhancement ratios for characterization of histopathologic grade. J Magn Reson Imaging 44:1432–1441
Fenwick SW, Wyatt JI, Toogood GJ, Lodge JP (2004) Hepatic resection and transplantation for primary carcinoid tumors of the liver. Ann Surg 239:210–219
Knox CD, Anderson CD, Lamps LW, Adkins RB, Pinson CW (2003) Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol 10:1171–1175
Kang TW, Rhim H, Lee J et al (2016) Magnetic resonance imaging with gadoxetic acid for local tumour progression after radiofrequency ablation in patients with hepatocellular carcinoma. Eur Radiol 26:3437–3446
Asbach P, Hein PA, Stemmer A et al (2008) Free-breathing echo-planar imaging based diffusion-weighted magnetic resonance imaging of the liver with prospective acquisition correction. J Comput Assist Tomogr 32:372–378
Haradome H, Grazioli L, Tsunoo M et al (2010) Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artifacts on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J Magn Reson Imaging 32:334–340
Tse JR, Naini BV, DS L, Raman SS (2016) Qualitative and Quantitative Gadoxetic Acid-enhanced MR Imaging Helps Subtype Hepatocellular Adenomas. Radiology 279:118–127
Ba-Ssalamah A, Antunes C, Feier D et al (2015) Morphologic and Molecular Features of Hepatocellular Adenoma with Gadoxetic Acid-enhanced MR Imaging. Radiology 277:104–113
Merkle EM, Zech CJ, Bartolozzi C et al (2016) Consensus report from the 7th International Forum for Liver Magnetic Resonance Imaging. Eur Radiol 26:674–682
Hood L, Friend SH (2011) Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat Rev Clin Oncol 8:184–187
Rudin M (2007) Imaging readouts as biomarkers or surrogate parameters for the assessment of therapeutic interventions. Eur Radiol 17:2441–2457
Fairweather M, Swanson R, Wang J et al (2017) Management of Neuroendocrine Tumor Liver Metastases: Long-Term Outcomes and Prognostic Factors from a Large Prospective Database. Ann Surg Oncol 24:2319–2325
Ramage JK, Ahmed A, Ardill J et al (2012) Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut 61:6–32
Wang H, Cruz-Roa A, Basavanhally A et al (2014) Mitosis detection in breast cancer pathology images by combining handcrafted and convolutional neural network features. J Med Imaging (Bellingham) 1:034003
Adesoye T, Daleo MA, Loeffler AG, Winslow ER, Weber SM, Cho CS (2015) Discordance of Histologic Grade Between Primary and Metastatic Neuroendocrine Carcinomas. Ann Surg Oncol 22(Suppl 3):S817–S821
Janson ET, Holmberg L, Stridsberg M et al (1997) Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol 8:685–690
Shen YH, Chen S, Zhang WT et al (2014) Clinical analysis of gastroenteropancreatic neuroendocrine tumor with liver metastasis, compared with primary hepatic neuroendocrine tumor. J Cancer Res Ther 10(Suppl):276–280
Yang Z, Tang LH, Klimstra DS (2011) Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol 35:853–860
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Won Jae Lee.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Insuk Sohn) has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Case-control
• Performed at one institution
Rights and permissions
About this article
Cite this article
Min, J.H., Kang, T.W., Kim, Y.K. et al. Hepatic neuroendocrine tumour: Apparent diffusion coefficient as a potential marker of prognosis associated with tumour grade and overall survival. Eur Radiol 28, 2561–2571 (2018). https://doi.org/10.1007/s00330-017-5248-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5248-3