Abstract
Objective
To evaluate the ability of quantitative dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to differentiate malignant from benign adnexal tumours.
Methods
Fifty-six women with 38 malignant and 18 benign tumours underwent MR imaging before surgery for complex adnexal masses. Microvascular parameters were extracted from high temporal resolution DCE-MRI series, using a pharmacokinetic model in the solid tissue of adnexal tumours. These parameters were tissue blood flow (FT), blood volume fraction (Vb), permeability-surface area product (PS), interstitial volume fraction (Ve), lag time (Dt) and area under the enhancing curve (rAUC). Area under the receiver operating curve (AUROC) was calculated as a descriptive tool to assess the overall discrimination of parameters.
Results
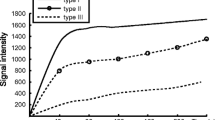
Malignant tumours displayed higher FT, Vb, rAUC and lower Ve than benign tumours (P < 0.0001, P = 0.0006, P = 0.04 and P = 0.0002, respectively). FT was the most relevant factor for discriminating malignant from benign tumours (AUROC = 0.86). Primary ovarian invasive tumours displayed higher FT and shorter Dt than borderline tumours. Malignant adnexal tumours with associated peritoneal carcinomatosis at surgery displayed a shorter Dt than those without peritoneal carcinomatosis at surgery (P = 0.01).
Conclusion
Quantitative DCE-MRI is a feasible and accurate technique to differentiate malignant from benign adnexal tumours and could potentially help oncologists with management decisions.
Key Points
• Quantitative DCE MR imaging allows accurate differentiation between malignant and benign tumours
• Quantitative DCE MRI may help predict peritoneal carcinomatosis associated with ovarian tumors
• Quantitative DCE MRI helps distinguish between invasive and borderline primary ovarian tumours




Similar content being viewed by others
References
Padhani AR, Dzik-Jurasz A (2004) Perfusion MR imaging of extracranial tumor angiogenesis. Top Magn Reson Imaging 15:41–57
O’Connor JP, Jackson A, Parker GJ et al (2007) DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer 96:189–195
Van Vierzen PB, Massuger LF, Ruys SH et al (1998) Borderline ovarian malignancy: ultrasound and fast dynamic MR findings. Eur J Radiol 28:136–142
Sohaib SA, Sahdev A, Van Trappen P et al (2003) Characterization of adnexal mass lesions on MR imaging. AJR Am J Roentgenol 180:1297–1304
Thomassin-Naggara I, Toussaint I, Perrot N et al (2011) Characterization of complex adnexal masses: value of adding perfusion- and diffusion-weighted MR imaging to conventional MR imaging. Radiology 258:793–803
Thomassin-Naggara I, Darai E, Cuenod CA et al (2008) Dynamic contrast-enhanced magnetic resonance imaging: a useful tool for characterizing ovarian epithelial tumors. J Magn Reson Imaging 28:111–120
Brix G, Semmler W, Port R et al (1991) Pharmacokinetic parameters in CNS Gd-DTPA enhanced MR imaging. J Comput Assist Tomogr 15:621–628
Tofts PS, Kermode AG (1991) Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med 17:357–367
Larsson HB, Stubgaard M, Sondergaard L et al (1994) In vivo quantification of the unidirectional influx constant for Gd-DTPA diffusion across the myocardial capillaries with MR imaging. J Magn Reson Imaging 4:433–440
Timmerman D, Valentin L, Bourne TH et al (2000) Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet Gynecol 16:500–505
Thomassin-Naggara I, Balvay D, Cuenod CA et al (2010) Dynamic contrast-enhanced MR imaging to assess physiologic variations of myometrial perfusion. Eur Radiol 20:984–994
Cenic A, Nabavi DG, Craen RA et al (2000) A CT method to measure hemodynamics in brain tumors: validation and application of cerebral blood flow maps. AJNR Am J Neuroradiol 21:462–470
Cenic A, Nabavi DG, Craen RA et al (1999) Dynamic CT measurement of cerebral blood flow: a validation study. AJNR Am J Neuroradiol 20:63–73
Brix G, Bahner ML, Hoffmann U et al (1999) Regional blood flow, capillary permeability, and compartmental volumes: measurement with dynamic CT—initial experience. Radiology 210:269–276
Pradel C, Siauve N, Bruneteau G et al (2003) Reduced capillary perfusion and permeability in human tumour xenografts treated with the VEGF signalling inhibitor ZD4190: an in vivo assessment using dynamic MR imaging and macromolecular contrast media. Magn Reson Imaging 21:845–851
De Bazelaire C, Siauve N, Fournier L et al (2005) Comprehensive model for simultaneous MRI determination of perfusion and permeability using a blood-pool agent in rats rhabdomyosarcoma. Eur Radiol 15:2497–2505
Balvay D, Tropres I, Billet R et al (2009) Mapping the zonal organization of tumor perfusion and permeability in a rat glioma model by using dynamic contrast-enhanced synchrotron radiation CT. Radiology 250:692–702
Balvay D, Frouin F, Calmon G et al (2005) New criteria for assessing fit quality in dynamic contrast-enhanced T1-weighted MRI for perfusion and permeability imaging. Magn Reson Med 54:868–877
Fleischer AC, Rodgers WH, Kepple DM et al (1993) Color Doppler sonography of ovarian masses: a multiparameter analysis. J Ultrasound Med 12:41–48
Abu-Jawdeh GM, Faix JD, Niloff J et al (1996) Strong expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in ovarian borderline and malignant neoplasms. Lab Invest 74:1105–1115
Yamamoto S, Konishi I, Mandai M et al (1997) Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer 76:1221–1227
Chen H, Ye D, Xie X et al (2004) VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma. Gynecol Oncol 94:630–635
Thomassin-Naggara I, Bazot M, Darai E et al (2008) Epithelial ovarian tumors: value of dynamic contrast-enhanced MR imaging and correlation with tumor angiogenesis. Radiology 248:148–159
Bazot M, Nassar-Slaba J, Thomassin-Naggara I et al (2006) MR imaging compared with intraoperative frozen-section examination for the diagnosis of adnexal tumors; correlation with final histology. Eur Radiol 16:2687–2699
Thomassin-Naggara I, Darai E, Cuenod CA et al (2009) Contribution of diffusion-weighted MR imaging for predicting benignity of complex adnexal masses. Eur Radiol 19:1544–1552
Bakir B, Bakan S, Tunaci M et al (2010) Diffusion-weighted imaging of solid or predominantly solid gynaecological adnexial masses: is it useful in the differential diagnosis? Br J Radiol 84:600–611
Takeuchi M, Matsuzaki K, Nishitani H (2010) Diffusion-weighted magnetic resonance imaging of ovarian tumors: differentiation of benign and malignant solid components of ovarian masses. J Comput Assist Tomogr 34:173–176
Fujii S, Matsusue E, Kanasaki Y et al (2008) Detection of peritoneal dissemination in gynecological malignancy: evaluation by diffusion-weighted MR imaging. Eur Radiol 18:18–23
Satoh Y, Ichikawa T, Motosugi U et al (2011) Diagnosis of peritoneal dissemination: comparison of 18F-FDG PET/CT, diffusion-weighted MRI, and contrast-enhanced MDCT. AJR Am J Roentgenol 196:447–453
Vergote I, Trope CG, Amant F et al (2010) Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363:943–953
Barentsz JO, Berger-Hartog O, Witjes JA et al (1998) Evaluation of chemotherapy in advanced urinary bladder cancer with fast dynamic contrast-enhanced MR imaging. Radiology 207:791–797
Padhani AR (2002) Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging 16:407–422
Padhani AR, Hayes C, Assersohn L et al (2006) Prediction of clinicopathologic response of breast cancer to primary chemotherapy at contrast-enhanced MR imaging: initial clinical results. Radiology 239:361–374
Padhani AR, Hayes C, Landau S et al (2002) Reproducibility of quantitative dynamic MRI of normal human tissues. NMR Biomed 15:143–153
Mitchell CL, O’Connor JP, Jackson A et al (2010) Identification of early predictive imaging biomarkers and their relationship to serological angiogenic markers in patients with ovarian cancer with residual disease following cytotoxic therapy. Ann Oncol 21:1982–1989
Padhani AR, Husband JE (2001) Dynamic contrast-enhanced MRI studies in oncology with an emphasis on quantification, validation and human studies. Clin Radiol 56:607–620
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 54 kb)
Rights and permissions
About this article
Cite this article
Thomassin-Naggara, I., Balvay, D., Aubert, E. et al. Quantitative dynamic contrast-enhanced MR imaging analysis of complex adnexal masses: a preliminary study. Eur Radiol 22, 738–745 (2012). https://doi.org/10.1007/s00330-011-2329-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-011-2329-6