Abstract
Backgrounds
Many patients with gastric cancer relapse during or early after adjuvant chemotherapy. The standard treatment for early relapse patients is a second-line chemotherapy (SLC) based on irinotecan, taxanes, or a platinum-based chemotherapy. The platinum-containing biweekly irinotecan plus cisplatin (IRI/CDDP) combination was assumed to be promising in several reports of clinical trials as SLC. TRICS trial, a randomized phase III study of IRI/CDDP vs. IRI in platinum-naïve gastric cancers refractory to S-1 monotherapy, revealed that both irinotecan-based chemotherapies were effective and well tolerated.
Methods
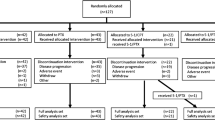
This study analyzed 108 patients in the TRICS trial who experienced early relapse. Patients receiving IRI/CDDP (IRI, 60 mg/m2; CDDP, 30 mg/m2, q2w) versus IRI (150 mg/m2, q2w) were compared regarding overall survival (OS), progression-free survival (PFS), overall response rate (ORR), and safety.
Results
The OS was 14.0 (95% confidence interval [CI]: 11.0–21.2) and 14.0 (95% CI: 10.7–16.5) months for IRI/CDDP and IRI, respectively (hazard ratio [HR]: 0.782; 95% CI: 0.515–1.188, P = 0.249). No significant differences were observed for PFS (5.0 vs. 4.5 months, respectively; HR: 0.802; 95% CI: 0.543–1.185, P = 0.268) or ORR (19.6% [95% CI: 9.4–33.9%] vs. 23.3% [95% CI: 11.8–38.6%], respectively). The incidence of grade 3–4 anemia was higher for IRI/CDDP than for IRI (20% vs. 0%, respectively; P = 0.0006).
Conclusion
Our study showed no significant survival differences between IRI/CDDP and IRI in platinum-naïve patients who relapsed during or within 6 months after S-1 adjuvant therapy; therefore, IRI may be a good option in this population.
Clinical trial information
UMIN 000002571.


Similar content being viewed by others
References
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Furukawa H, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T et al (2011) Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29:4387–4393
Ito S, Ohashi Y, Sasako M (2018) Survival after recurrence in patients with gastric cancer who receive S-1 adjuvant chemotherapy: exploratory analysis of the ACTS-GC trial. BMC Cancer 18(1):449. [Epub ahead of print]
Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T et al (2013) Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 31:4438–4444
Higuchi K, Tanabe S, Shimada K, Hosaka H, Sasaki E, Nakayama N et al (2014) Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: a randomised phase III trial (TCOG GI-0801/BIRIP trial). Eur J Cancer 50:1437–1445
Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J et al (2014) Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 15:78–86
Kang HJ, Chang HM, Kim TW, Ryu MH, Sohn HJ, Yook JH et al (2005) Phase II study of capecitabine and cisplatin as first-line combination therapy in patients with gastric cancer recurrent after fluoropyrimidine-based adjuvant chemotherapy. Br J Cancer 92:246–251
Park YH, Kim BS, Ryoo BY, Yang SH (2006) A phase II study of capecitabine plus 3-weekly oxaliplatin as first-line therapy for patients with advanced gastric cancer. Br J Cancer 94:959–963
De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S et al (2005) A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer 92:1644–1649
Nishikawa K, Fujitani K, Inagaki H, Akamaru Y, Tokunaga S, Takagi M et al (2015) Randomised phase III trial of second-line irinotecan plus cisplatin versus irinotecan alone in patients with advanced gastric cancer refractory to S-1 monotherapy: TRICS trial. Eur J Cancer 51:808–816
Sato A, Kurihara M, Matsukawa M, Shimada K, Yamazaki T, Nakamachi M et al (2001) Preliminary study of fortnightly irinotecan hydrochloride plus cisplatin therapy in patients with advanced gastric and colorectal cancer. Cancer Chemother Pharmacol 47:380–384
Koizumi W, Kurihara M, Satoh A, Takiuchi H, Tanabe S, Shimada K et al (2005) Phase I/II study of bi-weekly irinotecan plus cisplatin in the treatment of advavced gastric cancer. Anticancer Res 25:1257–1262
Shitara K, Morita S, Fujitani K, Kadowaki S, Takiguchi N, Hirabayashi N et al (2012) Combination chemotherapy with S-1 plus cisplatin for gastric cancer that recurs after adjuvant chemotherapy with S-1: multi-institutional retrospective analysis. Gastric Cancer 15:245–251
Nishikawa K, Tsuburaya A, Yoshikawa T, Takahashi M, Tanabe K, Yamaguchi K et al (2018) A phase II trial of capecitabine plus cisplatin (XP) for patients with advanced gastric cancer with early relapse after S-1 adjuvant therapy: XParTS-I trial. Gastric Cancer 21:811–818
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15:1224–1235
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH et al (2011) Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 379:315–321
Fuse N, Bando H, Chin K, Ito S, Yoshikawa T, Tsuburaya A et al (2017) Adjuvant capecitabine plus oxaliplatin after D2 gastrectomy in Japanese patients with gastric cancer: a phase II study. Gastric Cancer 20:332–340
Acknowledgements
We thank the investigators who enrolled patients in this trial. Furthermore, we deeply appreciate all patients who participated in the trial. This work was supported in part by the non-profit organization Epidemiological & Clinical Research Information Network (ECRIN).
Funding
This work was supported, in part, by the non-profit organization Epidemiological & Clinical Research Information Network (ECRIN). [No grant numbers apply].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kazuhiro Nishikawa has received honoraria from Chugai, Taiho, Yakult, Eli Lilly, Tsumura, and EA Pharma, and research funding from Yakult and Taiho, outside the submitted work. Takaki Yoshikawa has received lecture fees from Chugai, Taiho, Yakult, Eli Lilly and Ono, and for advisory work for Ono and MSD, outside the submitted work. Masato Nakamura has received honoraria from Chugai, Taiho, Merk, Takeda, Yakult, Eli Lilly, Bayer, Ono, and Otsuka Pharma, outside the submitted work. Satoshi Morita has received honoraria from Chugai and Taiho, outside the submitted work. Junichi Sakamoto has received consultant fee from Takeda, and Honoraria from Tsumura, Nihon Kayaku, and Chugai, outside the submitted work. All remaining authors have declared no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishikawa, K., Murotani, K., Fujitani, K. et al. A study of second-line irinotecan plus cisplatin vs. irinotecan alone in platinum-naïve patients with early relapse of gastric cancer refractory to adjuvant S-1 monotherapy: exploratory subgroup analysis of the randomized phase III TRICS trial. Cancer Chemother Pharmacol 83, 867–874 (2019). https://doi.org/10.1007/s00280-019-03802-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03802-9