Abstract
Purpose
Ewing’s sarcoma (ES) is a rare and recalcitrant disease which is in need of a development of a novel effective therapy. The aim of this study was to investigate the efficacy of regorafenib on an ES tumor in a patient-derived orthotopic xenograft (PDOX) model.
Methods
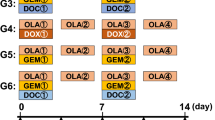
The ES PDOX models were established orthotopically in the right chest wall of nude mice to match the site of the tumor in the donor patient. The ES PDOX models were randomized into three groups (G) when the tumor volume reached 75 mm3: G1: untreated control; G2: doxorubicin (DOX) (i.p., 3 mg/kg, weekly, 2 weeks); G3: regorafenib (REG) (p.o., 30 mg/kg, daily, 2 weeks). Tumor volume and body weight were measured twice a week. All mice were sacrificed on day 15.
Results
DOX was ineffective compared to the control group (P = 0.229). REG regressed the tumor size (P < 0.001 and P < 0.001, relative to control and DOX, respectively).
Conclusions
Our findings suggest that REG has clinical potential for ES patients whose tumors respond to REG in a PDOX model.





Similar content being viewed by others
References
Balamuth NJ, Womer RB (2010) Ewing’s sarcoma. Lancet Oncol 11:184–192
Kovar H, Alonso J, Aman P, Aryee DN, Ban J, Burchill SA, Burdach S, De Alava E, Delattre O, Dirksen U, Fourtouna A, Fulda S, Helman LJ, Herrero-Martin D, Hogendoorn PC, Kontny U, Lawlor ER, Lessnick SL, Llombart-Bosch A, Metzler M, Moriggl R, Niedan S, Potratz J, Redini F, Richter GH, Riedmann LT, Rossig C, Schäfer BW, Schwentner R, Scotlandi K, Sorensen PH, Staege MS, Tirode F, Toretsky J, Ventura S, Eggert A, Ladenstein R (2012) The first European interdisciplinary ewing sarcoma research summit. Front Oncol 2:54
Paulussen M, Ahrens S, Burdach S, Craft A, Dockhorn-Dworniczak B, Dunst J, Fröhlich B, Winkelmann W, Zoubek A, Jürgens H (1998) Primary metastatic (stage IV) Ewing tumor: survival analysis of 171 patients from the EICESS studies. European Intergroup Cooperative Ewing Sarcoma Studies. Annals Oncol 9:275–281
Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D (2011) Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129:245–255
FDA approves (2012) regorafenib (Stivarga) for metastatic colorectal cancer. Oncology 26:896
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D, CORRECT Study Group (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:303–312
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L, Yeh KH, Bi F, Cheng Y, Le AT, Lin JK, Liu T, Ma D, Kappeler C, Kalmus J, Kim TW, CONCUR Investigators (2015) Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 16:619–629
Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, Hohenberger P, Leahy M, von Mehren M, Joensuu H, Badalamenti G, Blackstein M, Le Cesne A, Schöffski P, Maki RG, Bauer S, Nguyen BB, Xu J, Nishida T, Chung J, Kappeler C, Kuss I, Laurent D, Casali PG, GRID study investigators (2013) Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:295–302
Poh A (2017) Regorafenib approved for liver cancer. Cancer Discov 7:660
Duffy AG, Greten TF (2017) Liver cancer: Regorafenib as second-line therapy in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 14:141–142
Kim K, Jha R, Prins PA, Wang H, Chacha M, Hartley ML, He AR (2017) Regorafenib in advanced hepatocellular carcinoma (HCC): considerations for treatment. Cancer Chemother Pharmacol 80:945–954
Berry V, Basson L, Bogart E, Mir O, Blay JY, Italiano A, Bertucci F, Chevreau C, Clisant-Delaine S, Liegl-Antzager B, Tresch-Bruneel E, Wallet J, Taieb S, Decoupigny E, Le Cesne A, Brodowicz T, Penel N (2017) REGOSARC: regorafenib versus placebo in doxorubicin-refractory soft-tissue sarcoma-A quality-adjusted time without symptoms of progression or toxicity analysis. Cancer 123:2294–2302
Duffaud F, Mir O, Boudou-Rouquette P, Piperno-Neumann S, Penel N, Bompas E, Delcambre C, Kalbacher E, Italiano A, Collard O, Chevreau C, Saada E, Isambert N, Delaye J, Schiffler C, Bouvier C, Vidal V, Chabaud S, Blay JY; French Sarcoma Group (2019) Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol 20:120–133
Sun W, Patel A, Normolle D, Patel K, Ohr J, Lee JJ, Bahary N, Chu E, Streeter N, Drummond S (2018) A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer. https://doi.org/10.1002/cncr.31872
Lombardi G, De Salvo GL, Brandes AA, Eoli M, Rudà R, Faedi M, Lolli I, Pace A, Daniele B, Pasqualetti F, Rizzato S, Bellu L, Pambuku A, Farina M, Magni G, Indraccolo S, Gardiman MP, Soffietti R, Zagonel V (2019) Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol 20:110–119
Hu X, Wu LW, Zhang ZY, Chen ML, Li YL, Zhang C (2018) The anti-tumor effect of regorafenib in lung squamous cell carcinoma in vitro. Biochem Biophys Res Commun 503:1123–1129
Breitkreutz I, Podar K, Figueroa-Vazquez V, Wilhelm S, Hayden PJ, Anderson KC, Raab MS (2018) The orally available multikinase inhibitor regorafenib (BAY 73-4506) in multiple myeloma. Ann Hematol 97:839–849
Chen C, Choudhury S, Wangsa D, Lescott CJ, Wilkins DJ, Sripadhan P, Liu X, Wangsa D, Ried T, Moskaluk C, Wick MJ, Glasgow E, Schlegel R, Agarwal S (2017) A multiplex preclinical model for adenoid cystic carcinoma of the salivary gland identifies regorafenib as a potential therapeutic drug. Sci Rep 7:11410
Hoffman RM (2015) Patient-derived orthotopic xenografts: better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer 15:451–452
Murakami T, Singh AS, Kiyuna T, Dry SM, Li Y, James AW, Igarashi K, Kawaguchi K, DeLong JC, Zhang Y, Hiroshima Y, Russell T, Eckardt MA, Yanagawa J, Federman N, Matsuyama R, Chishima T, Tanaka K, Bouvet M, Endo I, Eilber FC, Hoffman RM (2016) Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing’s sarcoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget 7:47556–47564
Miyake K, Murakami T, Kiyuna T, Igarashi K, Kawaguchi K, Li Y, Singh AS, Dry SM, Eckardt MA, Hiroshima Y, Momiyama M, Matsuyama R, Chishima T, Endo I, Eilber FC, Hoffman RM (2018) Eribulin regresses a doxorubicin-resistant Ewing’s sarcoma with a FUS-ERG fusion and CDKN2A-deletion in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Cell Biochem 119:967–972
Miyake K, Murakami T, Kiyuna T, Igarashi K, Kawaguchi K, Miyake M, Li Y, Nelson SD, Dry SM, Bouvet M, Elliott IA, Russell TA, Singh AS, Eckardt MA, Hiroshima Y, Momiyama M, Matsuyama R, Chishima T, Endo I, Eilber FC, Hoffman RM (2017) The combination of temozolomide-irinotecan regresses a doxorubicin-resistant patient-derived orthotopic xenograft (PDOX) nude-mouse model of recurrent Ewing’s sarcoma with a FUS-ERG fusion and CDKN2A deletion: Direction for third-line patient therapy. Oncotarget 8:103129–103136
Daudigeos-Dubus E, Le Dret L, Lanvers-Kaminsky C, Bawa O, Opolon P, Vievard A, Villa I, Pagès M, Bosq J, Vassal G, Zopf D, Geoerger B (2015) Regorafenib: antitumor activity upon mono and combination therapy in preclinical pediatric malignancy models. PLoS One 10:e0142612
Murakami T, Kiyuna T, Kawaguchi K, Igarashi K, Singh AS, Hiroshima Y, Zhang Y, Zhao M, Miyake K, Nelson SD, Dry SM, Li Y, DeLong JC, Lwin TM, Chishima T, Tanaka K, Bouvet M, Endo I, Eilber FC, Hoffman RM (2017) The irony of highly-effective bacterial therapy of a patient-derived orthotopic xenograft (PDOX) model of Ewing’s sarcoma, which was blocked by Ewing himself 80 years ago. Cell Cycle 16:1046–1052
Wunder JS, Paulian G, Huvos AG, Heller G, Meyers PA, Healey JH (1998) The histological response to chemotherapy as a predictor of the oncological outcome of operative treatment of Ewing sarcoma. J Bone Jt Surg Am 80:1020–1033
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl 48:452–458
Miyake K, Kiyuna T, Miyake M, Zhao M, Wangsiricharoen S, Kawaguchi K, Zhang Z, Higuchi T, Razmjooei S, Li Y, Nelson SD, Russell T, Singh A, Murakami T, Hiroshima Y, Momiyama M, Matsuyama R, Chishima T, Singh SR, Chawla SP, Eilber FC, Endo I, Hoffman RM (2018) Tumor-targeting Salmonella typhimurium A1-R overcomes partial carboplatinum-resistance of a cancer of unknown primary (CUP). Tissue Cell 54:144–149
Kiyuna T, Tome Y, Murakami T, Kawaguchi K, Igarashi K, Miyake K, Miyake M, Li Y, Nelson SD, Dry SM, Singh AS, Russell TA, Elliott I, Singh SR, Kanaya F, Eilber FC, Hoffman RM (2018) Trabectedin arrests a doxorubicin-resistant PDGFRA-activated liposarcoma patient-derived orthotopic xenograft (PDOX) nude mouse model. BMC Cancer 18:840
Kawaguchi K, Miyake K, Han Q, Li S, Tan Y, Igarashi K, Kiyuna T, Miyake M, Higuchi T, Oshiro H, Zhang Z, Razmjooei S, Wangsiricharoen S, Bouvet M, Singh SR, Unno M, Hoffman RM (2018) Oral recombinant methioninase (o-rMETase) is superior to injectable rMETase and overcomes acquired gemcitabine resistance in pancreatic cancer. Cancer Lett 432:251–259
Igarashi K, Kawaguchi K, Li S, Han Q, Tan Y, Murakami T, Kiyuna T, Miyake K, Miyake M, Singh AS, Eckardt MA, Nelson SD, Russell TA, Dry SM, Li Y, Yamamoto N, Hayashi K, Kimura H, Miwa S, Tsuchiya H, Singh SR, Eilber FC, Hoffman RM (2018) Recombinant methioninase in combination with doxorubicin (DOX) overcomes first-line DOX resistance in a patient-derived orthotopic xenograft nude-mouse model of undifferentiated spindle-cell sarcoma. Cancer Lett 417:168–173
Li X, Tanaka K, Nakatani F, Matsunobu T, Sakimura R, Hanada M, Okada T, Nakamura T, Iwamoto Y (2005) Transactivation of cyclin E gene by EWS-Fli1 and antitumor effects of cyclin dependent kinase inhibitor on Ewing’s family tumor cells. Int J Cancer 116:385–394
Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G et al (1992) Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 359:162–165
Parham DM, Hijazi Y, Steinberg SM, Meyer WH, Horowitz M, Tzen CY, Wexler LH, Tsokos M (1999) Neuroectodermal differentiation in Ewing’s sarcoma family of tumors does not predict tumor behavior. Human Pathol 30:911–918
Pinto A, Dickman P, Parham D (2011) Pathobiologic markers of the ewing sarcoma family of tumors: state of the art and prediction of behaviour. Sarcoma 2011:856190
Attia S, Bolejack V, Ganjoo KN et al (2017) A phase II trial of regorafenib (REGO) in patients (pts) with advanced Ewing sarcoma and related tumors (EWS) of soft tissue and bone: SARC024 trial results. J Clin Oncol 35:11005
Acknowledgements
This paper is dedicated to the memory of A. R. Moossa, M.D., and Sun Lee, M.D.
Funding
This work was supported in part by a Yokohama City University research grant “KAMOME Project” which had no role in the research or writing the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. K.M., T.K., K.K., T.H., H.O., Z.Z., S.W., S.R., T.M., Y.H., and RMH are or were unsalaried associates of AntiCancer, Inc. AntiCancer, Inc. uses PDOX models for contract research.
Ethical approval
All experiments were performed with an AntiCancer Institutional Animal Care and Use Committee (IACUC)-protocol specifically approved for this study and in accordance with the principals and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals under Assurance Number A3873-1.
Informed consent
The patient signed an informed consent form. This study was approved by the Institutional Review Board of UCLA.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miyake, K., Kiyuna, T., Kawaguchi, K. et al. Regorafenib regressed a doxorubicin-resistant Ewing’s sarcoma in a patient-derived orthotopic xenograft (PDOX) nude mouse model. Cancer Chemother Pharmacol 83, 809–815 (2019). https://doi.org/10.1007/s00280-019-03782-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03782-w