Abstract
Purpose
To evaluate the efficacy and safety of combined gemcitabine and S-1 as first-line chemotherapy for patients with locally advanced or metastatic pancreatic cancer.
Methods
This study included patients who had been diagnosed with unresectable, locally advanced or metastatic adenocarcinoma arising from the pancreas, which was histologically or cytologically confirmed and involved at least 1 unidimensionally measurable lesion. The regimen consisted of intravenous 1,000 mg/m2 gemcitabine on day 1 and 8 combined with oral S-1 on days 1–14 every 21 days. The dosage of S-1 was based on the body surface area (BSA) as follows: 40 mg bid (total 80 mg/day) for a BSA of <1.25, 50 mg bid (total 100 mg/day) for a BSA of ≥1.25 but <1.5, and 60 mg bid (total 120 mg/day) for a BSA of ≥1.5. Treatment consisted of at least 2 courses unless rapid disease progression was noted. The primary end points were the response and disease control rates, and the secondary end points were toxicity and survival.
Results
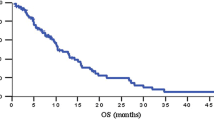
Thirty-seven patients were enrolled between August 2005 and December 2010. The median number of chemotherapy cycles was 4 (range 1–28 cycles). Response to treatment could be evaluated in 31 patients. None of the patients showed complete response, but 5 achieved partial response. The response rate was thus 13.5 % [95 % confidence interval (CI) 2.7–24.3 %] in the intent-to-treat population. Sixteen patients (43.2 %; 95 % CI 27–59.5 %) showed stable disease, and the overall disease control rate was 56.8 % (95 % CI 40.6–72.9 %). For all 37 patients, the median progression-free survival was 4.6 months (95 % CI 1.8–7.6 month), and the median overall survival was 9.4 month (95 % CI 5.8–12.6 month). Chemotherapy-related grade 3/4 hematological toxicities were neutropenia (36.1 %), leucopenia (22.2 %), and anemia (13.9 %). The non-hematological toxicities were generally mild.
Conclusions
Combination chemotherapy with gemcitabine and S-1 was effective, convenient, and safe in patients with advanced pancreatic cancer.



Similar content being viewed by others
References
Annual report of cancer statistics in Korea in 2009. National Cancer Control Institute
Mancuso A, Calabrò F, Sternberg CN (2006) Current therapies and advances in the treatment of pancreatic cancer. Crit Rev Oncol Hematol 58:231–241
Burris HA 3rd, Moore MJ, Andersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line chemotherapy for patients with advanced pancreatic cancer: a randomized trial. J Clin Oncol 15:2403–2413
Ozaka M, Matsumura Y, Ishii H et al (2012) Randomized phase II study of gemcitabine and S-1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan Clinical Cancer Research Organization PC-01 study). Cancer Chemother Pharmacol 69:1197–1204
Berlin JD, Catalano P, Thomas JP et al (2002) Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20:3270–3275
Colucci G, Giuliani F, Gebbia V et al (2002) Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic cancer. Cancer 94:902–910
Louvet C, André T, Lledo G et al (2002) Gemcitabine combined with oxaliplatin in advanced pancreatic adenocarcinoma: final results of a GERCOR multicenter phase II study. J Clin Oncol 20:1512–1528
Moore MJ, Goldstein D, Hamm J, National Cancer Institute of Canada Clinical Trial Group et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trial Group. J Clin Oncol 25:1960–1966
Shirasaka T, Nakano K, Takechi T et al (1996) Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 56:2602–2606
Okusaka T, Funakoshi A, Furuse J et al (2008) A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol 61:615–621
Ueno H, Okusaka T, Ikeda M et al (2005) An early phase II study of S-1 in patients with metastatic pancreatic cancer. Oncology 68:171–178
Nakamura K, Yamaguchi T, Ishihara T et al (2005) Phase I trial of oral S-1 combined with gemcitabine in metastatic pancreatic cancer. Br J Cancer 92:2134–2139
Nakamura K, Yamaguchi T, Ishihara T et al (2006) Phase II trial of oral S-1 combined with gemcitabine in metastatic pancreatic cancer. Br J Cancer 94:1575–1579
Ren Q, Kao V, Grem JL (1998) Cytotoxicity and DNA fragmentation associated with sequential gemcitabine and 5-fluoro-2′-deoxyuridine in HT-29 colon cancer cells. Clin Cancer Res 4(11):2811–2818
Hidalgo M, Castellano D, Paz-Ares L et al (1999) Phase I-II study of gemcitabine and fluorouracil as a continuous infusion in patients with pancreatic cancer. J Clin Oncol 17:585–592
Scheithauer W, Schüll B, Ulrich-Pur H et al (2003) Biweekly high-dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: a randomized phase II trial. Ann Oncol 14:97–104
Stathopoulos GP, Syrigos K, Polyzos A et al (2004) Front-line treatment of inoperable or metastatic pancreatic cancer with gemcitabine and capecitabine: an intergroup, multicenter, phase II study. Ann Oncol 15(2):224–229
Nakai Y, Isayama H, Sasaki T et al (2010) Impact of S-1 in patients with gemcitabine-refractory pancreatic cancer in Japan. Jpn J Clin Oncol 40:774–780
Ueno H, Ioka T, Ikeda M et al (2013) Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 1:1640–1648
Oh DY, Cha Y, Choi IS et al (2010) A multicenter phase II study of gemcitabine and S-1 combination chemotherapy in patients with unresectable pancreatic cancer. Cancer Chemother Pharmacol 65:527–536
Kim MK, Lee KH, Jang BI et al (2009) S-1 and gemcitabine as an outpatient-based regimen in patients with advanced or metastatic pancreatic cancer. Jpn J Clin Oncol 39:49–53
Ueno H, Okusaka T, Furuse J et al (2011) Multicenter phase II study of gemcitabine and S-1 combination therapy (GS therapy) in patients with metastatic pancreatic cancer. Jpn J Clin Oncol 41:953–958
Nakai Y, Isayama H, Sasaki T et al (2012) A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S-1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br J Cancer 106:1934–1939
Inal A, Kos FT, Algin E, Anatolian Society of Medical Oncology et al (2012) Prognostic factors in patients with advanced pancreatic cancer treated with gemcitabine alone or gemcitabine plus cisplatin: retrospective analysis of a multicenter study. J BUON 17:102–105
Yi JH, Lee J, Park SH et al (2011) A prognostic model to predict clinical outcomes with first-line gemcitabine-based chemotherapy in advanced pancreatic cancer. Oncology 80:175–180
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Acknowledgments
This trial was supported by Jeil Pharmaceutical Co. Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, H., Han, B., Park, C.K. et al. Phase II trial of gemcitabine and S-1 for patients with advanced pancreatic cancer. Cancer Chemother Pharmacol 72, 845–852 (2013). https://doi.org/10.1007/s00280-013-2265-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2265-z