Abstract
Purpose
To evaluate the response to lapatinib, an inhibitor of epidermal growth factor receptors 1 and 2, in patients with advanced bilary tree cancer (BTC) and hepatocellular cancer (HCC).
Methods
Lapatinib was dosed at 1,500 mg/day orally continuously.
Results
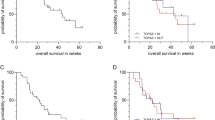
Fifty-seven patients were accrued (BTC 17, HCC 40). Therapy was well tolerated. The response in BTC was 0% and in HCC was 5%. The progression free survival (PFS) for BTC and HCC patients was 1.8 (95% CI: 1.7–5.2) months and 2.3 (95% CI: 1.7–5.6) months. The median survival for BTC and HCC patients was 5.2 (95% CI 3.3–∞) months and 6.2 (95% CI: 5.1–∞) months. EGFR genotyping indicated HCC patients with <20 repeats have the lowest PFS. The occurrence of any skin rash significantly prolonged PFS and survival.
Conclusions
Lapatinib was well-tolerated. There was evidence of activity in HCC, but therapy with lapatinib did not meet the predefined efficacy rate.


Similar content being viewed by others
References
El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132:2557–2576
Alberts SR, Gores GJ, Kim GP et al (2007) Treatment options for hepatobiliary and pancreatic cancer. Mayo Clin Proc 82:628–637
Shimoda M, Kubota K (2007) Multi-disciplinary treatment for cholangiocellular carcinoma. World J Gastroenterol 13:1500–1504
Bartlett D, Ramanathan RK, Ben-Joseph E (2008) Gallbladder and bilary cancers. In: Devita VT, Hellman S, Rosenberg SA (eds) Cancer: principles and practice of oncology, 8th edn. Lippincott, Philadelphia
Berasain C, Castillo J, Prieto J et al (2007) New molecular targets for hepatocellular carcinoma: the ErbB1 signaling system. Liver Int 27:174–185
Bekaii-Saab T, Williams N, Plass C et al (2006) A novel mutation in the tyrosine kinase domain of ERBB2 in hepatocellular carcinoma. BMC Cancer 6:278
Yoon JH, Gwak GY, Lee HS et al (2004) Enhanced epidermal growth factor receptor activation in human cholangiocarcinoma cells. J Hepatol 41:808–814
Sirica A (2005) Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology 41:5–15
Philip PA, Mahoney MR, Allmer C et al (2005) Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol 23:6657–6663
Thomas MB, Chadha R, Glover K et al (2007) Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer 110:1059–1067
Philip PA, Mahoney MR, Allmer C et al (2006) Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 24:3069–3074
Reid A, Vidal L, Shaw H et al (2007) Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu). Eur J Cancer 43:481–489
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Simon R (1989) Optimal two-stage design for phase II clinical trials. Control Clin Trials 10:1–10
Gautschi O, Huegli B, Ziegler A et al (2007) Origin and prognostic value of circulating KRAS mutations in lung cancer patients. Cancer Lett 254:265–273
Zhang W, Park DJ, Lu B et al (2005) Epidermal growth factor receptor gene polymorphisms predict pelvic recurrence in patients with rectal cancer treated with chemoradiation. Clin Cancer Res 11:600–605
Matsuo M, Sakurai H, Saiki I (2003) ZD1839, a selective epidermal growth factor receptor tyrosine kinase inhibitor, shows antimetastatic activity using a hepatocellular carcinoma model. Mol Cancer Ther 2:557–561
Schiffer E, Housset C, Cacheux W et al (2005) Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology 41:307–314
Zhu AX, Stuart K, Blaszkowsky LS et al (2007) Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer 110:581–589
Llovet JM, Ricci S, Mazzaferro V, SHARP Investigators Study Group (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Peréz-Soler R, Saltz L (2005) Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol 23:5235–5246
Geyer CE, Forster J, Lindquist D et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743
Press MF, Finn RS, Cameron D et al (2008) HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clin Cancer Res 14:7861–7870
Van Cutsem E, Lang I, D’haens G et al (2008) KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: the CRYSTAL experience. J Clin Oncol 26(May 20 suppl), abstr 2
Weihrauch M, Benicke M, Lehnert G et al (2001) Frequent k-ras-2 mutations and p16INK4Amethylation in hepatocellular carcinomas in workers exposed to vinyl chloride. Br J Cancer 84:982–989
Mayoral R, Fernandez-Martinez A, Bosca L et al (2005) Prostaglandin E2 promotes migration and adhesion in hepatocellular carcinoma cells. Carcinogenesis 26:753–761
Mitchell C, Nivison M, Jackson LF et al (2005) Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem 280:2562–2568
Buerger H, Gebhardt F, Schmidt H et al (2000) Length and loss of heterozygosity of an intron 1 polymorphic sequence of egfr is related to cytogenetic alterations and epithelial growth factor receptor expression. Cancer Res 60:854–857
Gebhardt F, Zanker KS, Brandt B (1999) Modulation of epidermal growth factor receptor gene transcription by a polymorphic dinucleotide repeat in intron 1. J Biol Chem 274:13176–13180
Llovet JM, Di Bisceglie AM, Bruix J et al (2008) Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 100:698–711
Acknowledgments
This study was sponsored by the Cancer Therapy and Evaluation Program of the National Cancer Institute, Bethesda, MD 20892. Presented in part at the 42nd annual meeting of the American Society of Clinical Oncology, Orlando, FL. June 2006. Supported in part by NCI-NO1-CM-57018-16 (California Consortium), Dhont Foundation (HJ Lenz). P30CA47904 and NIH/NCCR/GCRC #5M01 RR 00056 (University of Pittsburgh Cancer Institute and Medical Center). The authors wish to thank Christine Garcia and Stella Chen, CCC-P coordinators for data management and coordination of study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramanathan, R.K., Belani, C.P., Singh, D.A. et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol 64, 777–783 (2009). https://doi.org/10.1007/s00280-009-0927-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-0927-7