Abstract
Purpose
To determine the growth inhibitory effects of mifepristone on endometrial cancer cell growth and evaluate its effect on apoptosis using HEC-1-A and Ishikawa human endometrial cancer cell lines.
Methods
The human endometrial cancer cell lines, HEC-1-A and Ishikawa, were cultured in vitro. MTT assays were completed in order to estimate the IC50 of mifepristone. Both cell lines were then treated with the respective IC50 values. Immunohistochemistry assays were performed to investigate the expression of estrogen receptors alpha and beta (ERα/β), progesterone receptor alpha and beta (PR α/β), cyclooxygenase-2 (COX-2), bax, p53, and bcl-2. Flow cytometry analysis was performed to study cell cycle arrest and apoptosis.
Results
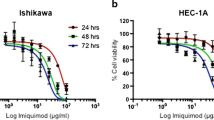
The estimated IC50 of mifepristone for HEC-1-A and Ishikawa was found to be 16 and 19 μg/ml respectively. At this concentration, there was no change in either ERα/β or PR α/β in Ishikawa. However, PR β expression increased with time of treatment in HEC-1-A. Expression of p53 was increased with duration of treatment in both cell lines. Consequently a decrease in bcl-2 and an increase in COX-2 expression were seen in HEC-1-A and Ishikawa cells, respectively. Lastly, flow cytometry analysis confirmed an accumulation of cells in G0 phase after 72 h of treatment in both cell lines.
Conclusions
Mifepristone demonstrates activity in both HEC-1-A and Ishikawa cells at clinically relevant concentrations based on an oral human dose of about 200 mg/day. While its mechanism of action remains unknown, this data supports an increase in apoptosis that may be due to p53 activation rather than hormone receptor mediation. Additional studies are needed to help further identify mifepristone mechanism of action.


Similar content being viewed by others
References
Jemal A, Siegel R, Ward E et al (2007) Cancer statistics, 2006. CA Cancer J Clin 57:43–66
Ellenson LH, Wu TC (2004) Focus on endometrial and cervical cancer. Cancer Cell 5:533–538
Humber C, Tierney J, Symonds P et al (2005) Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma. Cochrane Database Syst Rev (4):CD003915
Kaaks R, Lukanova A, Kurzer MS (2002) Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev 11:1531–1543
Gadducci A, Gargini A, Palla E, Fanuchhi A, Genazzani AR (2005) Polycystic ovary syndrome and gynecological cancers: is there a link? Gynecol Endocrinol 20:200–208
Danco Laboratories LLC (2005) Mifeprex (mifepristone) package insert. New York
Spitz IM (2003) Progesterone antagonists and progesterone receptor modulators: an overview. Steroids 68:981–993
Spitz IM, Robbins A (1998) Mechanism of action and clinical effects of antiprogestins on the non-pregnant uterus. Hum Reprod Update 4:584–593
Steinauer J, Pritts EA, Jackson R, Jacoby AF (2004) Systematic review of mifepristone for the treatment of uterine leiomyomata. Obstet Gynecol 103:1331–1336
Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS (2006) Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size. Obstet Gynecol 108:1381–1387
Mahajan DK, London SN (1997) Mifepristone (RU486): a review. Fertil Steril 68:967–976
Eisinger SH, Meldrum S, Fiscella K, le Roux HD, Guzick DS (2003) Low-dose mifepristone for uterine leiomyomata. Obstet Gynecol 101:243–250
Han S, Sidell N (2003) RU486-induced growth inhibition of human endometrial cells involves the nuclear factor-kappa B signaling pathway. J Clin Endocrinol Metab 88:713–719
Jiang J, Wu R, Wang Z, Sun H, Xu Z, Xiu H (2002) Effect of mifepristone on estrogen and progesterone receptors in human endometrial and endometriotic cells in vitro. Fertil Steril 77:995–1000
Li A, Felix JC, Minoo P, Amezcua CA, Jain JK (2005) Effect of mifepristone on proliferation and apoptosis of Ishikawa endometrial adenocarcinoma cells. Fertil Steril 84:202–211
Schneider CC, Gibb RK, Taylor DD, Wan T, Gercel-Taylor C (1998) Inhibition of endometrial cancer cell lines by mifepristone (RU486). J Soc Gynecol Invest 5:334–338
Mirkin S, Archer DF (2004) Effects of mifepristone on vascular endothelial growth factor and thrombospondin-1 mRNA in Ishikawa cells: implication for the endometrial effects of mifepristone. Contraception 70:327–333
Kamradt MC, Mohideen N, Vaughan AT (2000) RU486 increases radiosensitivity and restores apoptosis through modulation of HPV E6/E7 in dexamethasone-treated cervical carcinoma cells. Gynecol Oncol 77:177–182
Smith JA, Gaikwad A, Ramondetta LM, Wolf JK, Brown J (2006) Determination of the mechanism of gemcitabine modulation of cisplatin drug resistance in panel of human endometrial cancer cell lines. Gynecol Oncol 103:518–522
Murphy AA, Zhou MH, Malkapuram S, Santanam N, Parthasarathy S, Sidell N (2000) RU486-induced growth inhibition of human endometrial cells. Fertil Steril 74:1014–1019
Liang Y, Hou M, Kallab AM, Barrett JT, El Etreby F, Schoenlein PV (2003) Induction of antiproliferation and apoptosis in estrogen receptor negative MDA-231 human breast cancer cells by mifepristone and 4-hydroxytamoxifen combination therapy: a role for TGFbeta1. Int J Oncol 23:369–380
Lin VC-L, Aw SE, Ng EG, Ng EH-L, Tan MG-K (2001) Demonstration of mixed properties of RU486 in progesterone receptor (PR)-transfected MDA-MB-231 cells: a model for studying the functions of progesterone analogues. Br J Cancer 85:1978–1986
Gaddy VT, Barrett JT, Delk JN, Kallab AM, Porter AG, Schoenlein (2004) Mifepristone induces growth arrest, caspase activation, and apoptosis of estrogen receptor-expressing, antiestrogen-resistant breast cancer cells. Clin Cancer Res 10:5215–5225
Lessey BA, Ilesanmi AO, Castelbaum AJ et al (1996) Characterization of the functional progesterone receptor in an endometrial adenocarcinoma cell line (Ishikawa): progesterone-induced expression of the α1 integrin. Steroid Biochem Molec Biol 59:31–39
Bertagna X, Bertagna C, Luton JP, Husson JM, Girard F (1984) The new steroid analog RU 486 inhibits glucocorticoid action in man. J Clin Endocrinol Metab 59:25–28
Rae MT, Niven D, Critchley H, Harlow CR, Hillier SG (2004) Antiinflammatory steroid action in human ovarian surface epithelial cells. J Clin Endocrinol Metab 89:4538–4544
Chivers JE, Cambridge LM, Catley MC et al (2004) Differential effects of RU486 reveal distinct mechanisms for glucocorticoid repression of prostaglandin E release. Eur J Biochem 271:4042–4052
Rose FV, Barnea ER (1996) Response of human ovarian carcinoma cell lines to antiprogestin mifepristone. Oncogen 12:999–1003
Sun Y (2006) p53 and its downstream proteins as molecular targets of cancer. Mol Carcinog 45:409–415
Skildum A, Faivre E, Lange CA (2005) Progesterone receptors induce cell cycle progression via activation of mitogen-activated protein kinases. Mol Endocrinol 19:327–329
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Navo, M.A., Smith, J.A., Gaikwad, A. et al. In vitro evaluation of the growth inhibition and apoptosis effect of mifepristone (RU486) in human Ishikawa and HEC1A endometrial cancer cell lines. Cancer Chemother Pharmacol 62, 483–489 (2008). https://doi.org/10.1007/s00280-007-0628-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0628-z