Abstract
Purpose
Docetaxel is a semisynthetic taxane derived from the needles of the European yew (Taxus baccata) and it is an important chemotherapeutic agent in the treatment of recurrent ovarian, breast and non-small-cell lung cancers. Traditional dosing regimens with docetaxel involve doses of 60–100 mg/m2 by infusion every 3 weeks. Now weekly low-dose (30–36 mg/m2) regimens are being evaluated in phase I trials. Such low-dose studies require a more sensitive, specific and rapid assay of docetaxel in biological fluids for the determination of pharmacokinetic parameters. Because docetaxel is primarily metabolized by CYP3A4 and is highly protein-bound in the plasma, there is potential for drug-drug interactions and high interpatient variability in pharmacokinetics. Therefore, pharmacokinetic studies are an important component to understanding the therapeutic variability of docetaxel-containing chemotherapeutic regimens.
Methods
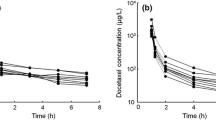
To this end, we developed an analytical assay for docetaxel based upon tandem LCMS and paclitaxel as an internal standard. The sensitivity of the new assay allowed us to monitor plasma levels of docetaxel out to 48 h after the end of the infusion in patients enrolled in a phase I trial of exisulind (orally, twice daily) receiving weekly docetaxel doses of 30 or 36 mg/m2 where plasma docetaxel levels are below the lower limit of quantitation for traditional HPLC/UV-based assays at later time-points.
Results
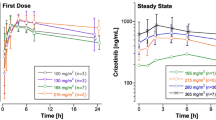
The inclusion of the 48-h time-point had significant effects on the calculated pharmacokinetic parameters when using either a three-compartment or non-compartmental analysis. The terminal half-life was significantly increased when the 48-h time-point was included in the pharmacokinetic analysis, and the use of model parameters derived with the inclusion of the 48-h time-point were able to more accurately predict plasma levels at later times.
Conclusions
The results reflect the importance of accurate and sensitive analytical methods for the determination of pharmacokinetic parameters and the effect of this later time-point on docetaxel pharmacokinetic modeling. Further, with the increased use of weekly docetaxel in combination with other agents, the inclusion of these later sampling time-points and sensitive methods for drug level determinations are important components in the description of pharmacokinetic drug interactions.






Similar content being viewed by others
References
Beer TM, Pierce WC, Lowe BA, Henner WD (2001) Phase II study of weekly docetaxel in symptomatic androgen-independent prostate cancer. Ann Oncol 12:1273
Burstein HJ, Manola J, Younger J, Parker LM, Bunnell CA, Scheib R, Matulonis UA, Garber JE, Clarke KD, Shulman LN, Winer EP (2000) Docetaxel administered on a weekly basis for metastatic breast cancer. J Clin Oncol 18:1212
Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D'Incalci M, Zucchetti M, Marmiroli P, Tredici G (2000) Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology 21:389
Clarke SJ, Rivory LP (1999) Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet 36:99
Copur MS, Ledakis P, Lynch J, Hauke R, Tarantolo S, Bolton M, Norvell M, Muhvic J, Hake L, Wendt J (2001) Weekly docetaxel and estramustine in patients with hormone-refractory prostate cancer. Semin Oncol 28:16
Fountzilas G, Tsavdaridis D, Kalogera-Fountzila A, Christodoulou CH, Timotheadou E, Kalofonos CH, Kosmidis P, Adamou A, Papakostas P, Gogas H, Stathopoulos G, Razis E, Bafaloukos D, Skarlos D (2001) Weekly paclitaxel as first-line chemotherapy and trastuzumab in patients with advanced breast cancer. A Hellenic Cooperative Oncology Group phase II study. Ann Oncol 12:1545
Garg MB, Ackland SP (2000) Simple and sensitive high-performance liquid chromatography method for the determination of docetaxel in human plasma or urine. J Chromatogr B 748:383
Gasparini G (2001) Metronomic scheduling: the future of chemotherapy? Lancet Oncol 2:733
Gianni L, Kearns CM, Giani A, Capri G, Vigano L, Lacatelli A, Bonadonna G, Egorin MJ (1995) Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol 13:180
Gustafsson LL, Ebling WF, Osaki E, Harapat S, Stanski DR, Shafer SL (1992) Plasma concentration clamping in the rat using a computer-controlled infusion pump. Pharm Res 9:800
Hainsworth JD, Burris HA, Erland JB, Thomas M, Greco FA (1998) Phase I trial of docetaxel administered by weekly infusion in patients with advanced refractory cancer. J Clin Oncol 16:2164
Hanahan D, Bergers G, Bergsland E (2000) Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest 105:1045
Huizing MT, Misser VH, Pieters RC, ten Bokkel Huinink WW, Veenhof CH, Vermorken JB, Pinedo HM, Beijnen JH (1995) Taxanes: a new class of antitumor agents. Cancer Invest 13:381
Kamen BA, Rubin E, Aisner J, Glatstein E (2000) High-time chemotherapy of high time for low dose. J Clin Oncol 18:2935
Kerns EH, Hill SE, Detlefsen DJ, Volk KJ, Long BH, Carboni J, Lee MS (1998) Cellular uptake profile of paclitaxel using liquid chromatography tandem mass spectrometry. Rapid Commun Mass Spectrom 12:620
Leca F, Marchiset-Leca D, Noble A, Antonetti M (1991) New data on the pharmacokinetics of adriamycin and its major metabolite, adriamycinol. Eur J Drug Metab Pharmacokinet 16:107
Loesch D, Robert N, Asmar L, Gregurich MA, O'Rourke M, Dakhil S, Cox E (2002) Phase II multicenter trial of a weekly paclitaxel and carboplatin regimen in patients with advanced breast cancer. J Clin Oncol 20:3857
Pectasides D, Glotsos J, Bountouroglou N, Kouloubinis A, Mitakidis N, Karvounis N, Ziras N, Athanassiou A (2002) Weekly chemotherapy with docetaxel, gemcitabine and cisplatin in advanced transitional cell urothelial cancer: a phase II trial. Ann Oncol 13:243
Riviere JE (1999) Study design and data analysis. In: Riviere JE (ed) Comparative pharmacokinetics: principles, techniques and applications. Iowa State University Press, Ames, p 239
Rosing H, Lustig V, Koopman FP, ten Bokkel Huinink WW, Beijnen JH (1997) Bio-analysis of docetaxel and hydroxylated metabolites in human plasma by high-performance liquid chromatography and automated solid-phase extraction. J Chromatogr B 696:89
Schellen A, Ooms B, van Gils M, Halmingh O, van der Vlis E, van de Lagemaat D, Verheij E (2000) High throughput on-line solid phase extraction/tandem mass spectrometric determination of paclitaxel in human serum. Rapid Commun Mass Spectrom 14:230
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtube assembly in vitro by taxol. Nature 277:665
Schwonzen M, Kurbacher CM, Mallmann P (2000) Liposomal doxorubicin and weekly paclitaxel in the treatment of metastatic breast cancer. Anticancer Drugs 11:681
Seidman AD, Hudis CA, Albanel J, Tong W, Tepler I, Currie V, Moynahan ME, Theodoulou M, Gollub M, Baselga J, Norton L (1998) Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol 16:3353
Sekine I, Nishiwaki Y, Watanabe K, Yoneda S, Saijo N (1996) Phase II study of 3-hour infusion of paclitaxel in previously untreated non-small cell lung cancer. Clin Cancer Res 2:941
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503
Socinski MA, Schell MJ, Bakri K, Peterman A, Lee J-H, Unger P, Yates S, Hudgens S, Kies MS (2002) Second-line, low-dose, weekly paclitaxel in patients with stage IIIB/IV nonsmall cell lung carcinoma who fail first-line chemotherapy with carboplatin plus paclitaxel. Cancer 95:1265
Sonnichsen DS, Relling MV (1998) Paclitaxel and docetaxel. In: Grochow LB, Ames MM (eds) A clinician's guide to chemotherapy pharmacokinetics and pharmacodynamics. Lippincott, Williams and Wilkins, Baltimore, p 375
Sonnichsen DS, Hurwitz CA, Pratt CB, Shuster JJ, Relling MV (1994) Saturable pharmacokinetics and paclitaxel pharmacodynamics in children with solid tumors. J Clin Oncol 12:532
Sottani C, Minoia C, D'Incalci M, Paganini M, Zucchetti M (1998) High-performance liquid chromatography tandem mass spectrometry procedure with automated solid phase extraction sample preparation for the quantitative determination of paclitaxel (Taxol) in human plasma. Rapid Commun Mass Spectrom 12:251
Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, Sledge GW Jr (2001) The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res 61:3369
Vergniol JC, Bruno R, Montay G, Frydman A (1992) Determination of taxotere in human plasma by a semi-automated high-performance liquid chromatographic method. J Chromatogr A 582:273
Wagner JG (1993) Noncompartmental and system analysis. In: Pharmacokinetics for the pharmaceutical scientist. Technomic Publishing Company, Lancaster, PA, p 83
Acknowledgements
This work was supported by CA75955 from the NCI to D.L.G. and by the University of Colorado Cancer Center Core grant (principle investigator: Dr. Paul A. Bunn, Jr.). The phase I clinical trial of docetaxel and exisulind in combination was supported by Aventis (Bridgewater, N.J.) and Cell Pathways (Horsham, Pa.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gustafson, D.L., Long, M.E., Zirrolli, J.A. et al. Analysis of docetaxel pharmacokinetics in humans with the inclusion of later sampling time-points afforded by the use of a sensitive tandem LCMS assay. Cancer Chemother Pharmacol 52, 159–166 (2003). https://doi.org/10.1007/s00280-003-0622-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0622-z