Abstract
Purpose
Cytotoxic T lymphocytes (CTL)- and T-helper cell-specific, and major histocompatibility complex (MHC) class-I and class-II peptides, respectively, of the HER-2/neu protein, induce immune responses in patients. A major challenge in developing cancer peptide vaccines is breaking tolerance to tumor-associated antigens which are functionally self-proteins. An adequate CD4+ T-helper response is required for effective and lasting responses.
Methods
Stimulating anti-cancer CD4+ T cell responses by MHC class-II epitope peptides has been limited by their weak potency, at least compared with tight-binding MHC class-I epitope peptides. Previously, a potent T-cell response to a MHC class-II epitope was engineered by coupling the N-terminus of the pigeon cytochrome C [PGCC(95–104)] MHC class-II epitope to the C-terminus of an immunoregulatory segment of the Ii protein (hIi77–81, the Ii-Key peptide) through a polymethylene spacer.
Results
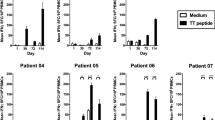
In vitro presentation of the MHC class-II epitope to a T hybridoma was enhanced greatly (>250 times). Now, an Ii-Key/HER-2/neu (777–789) MHC class-II epitope hybrid peptide stimulated lymphocytes from both a healthy donor and a patient with metastatic breast carcinoma. The in vitro primary stimulation with the hybrid peptide strongly activated IFN-γ release, whereas the epitope-only peptide was weakly active. In fact, the hybrid stimulated IFN-γ release as well as the wild-type peptide when augmented with IL-12; however, the hybrid was comparable to free peptide in stimulating IL-4 release. This pattern is consistent with preferential activation along a non-tolerogenic Th1 pathway.
Conclusion
Such Ii-Key/MHC class-II epitope hybrid peptides have both diagnostic and therapeutic applications.

Similar content being viewed by others
References
Adams S, Humphreys RE (1995) Invariant-chain peptides enhancing or inhibiting the presentation of antigenic peptides by major histocompatibility complex class-II molecules. Eur J Immunol 25:1693
Adams S, Albericio F, Alsina J, Smith ER, Humphreys RE (1997) Biological activity and therapeutic potential of homologs of an Ii peptide which regulates antigenic peptide binding to cell surface MHC class-II molecules. Arzneimittelforschung 47:1069
Anderson BW, Kudelka AP, Honda T, Pollack MS, Gershenson DM, Gillogly MA, Murray JL, Ioannides CG (2000) Induction of determinant spreading and of Th1 responses by in vitro stimulation with HER-2 peptides. Cancer Immunol Immunother 49:459
Anderson BW, Peoples GE, Murray JL, Gillogly MA, Gershenson DM, Ioannides CG (2000) Peptide priming of cytolytic activity to HER-2 epitope 369–377 in healthy individuals. Clin Cancer Res 6:4192
Bernhard H, Salazar L, Schiffman K, Smorlesi A, Schmidt B, Knutson KL, Disis ML (2002) Vaccination against the HER-2/neu oncogenic protein. Endocr Relat Cancer 9:33
Carr JA, Havstad S, Zarbo RJ, Divine G, Mackowiak P, Velanovich V (2000) The association of HER-2/neu amplification with breast cancer recurrence. Arch Surg 135:1469
Chen L, Chen D, Block E, O’Donnell M, Kufe DW, Clinton SK (1997) Eradication of murine-bladder carcinoma by intratumor injection of a bicistronic adenoviral vector carrying cDNAs for the IL-12 heterodimer and its inhibition by the IL-12 p40 subunit homodimer. J Immunol 159:351
Cibotti R, Kanellopoulos JM, Cabaniols JP, Halle-Panenko O, Kosmatopoulos K, Sercarz E, Kourilsky P (1992) Tolerance to a self-protein involves its immunodominant but does not involve its subdominant determinants. Proc Natl Acad Sci USA 89:416
De Placido S, Gallo C, Perrone F, Marinelli A, Pagliarulo C, Carlomagno C, Petrella G, D’Istria M, Delrio G, Bianco AR (1990) Prolactin receptor does not correlate with oestrogen and progesterone receptors in primary breast cancer and lacks prognostic significance. Ten year results of the Naples adjuvant (GUN) study. Br J Cancer 62:643
Disis ML, Calenoff E, McLaughlin G, Murphy AE, Chen W, Groner B, Jeschke M, Lydon N, McGlynn E, Livingston RB (1994) Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res 54:16
Disis ML, Bernhard H, Shiota FM, Hand SL, Gralow JR, Huseby ES, Gillis S, Cheever MA (1996) Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood 88:202
Disis ML, Gralow JR, Bernhard H, Hand SL, Rubin WD, Cheever MA (1996) Peptide-based, but not whole-protein, vaccines elicit immunity to HER-2/neu, oncogenic self-protein. J Immunol 156:3151
Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA (1997) High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol 15:3363
Disis ML, Knutson KL, Schiffman K, McNeel DG (2000) Pre-existent immunity to the HER-2/neu oncogenic protein in patients with HER-2/neu overexpressing breast and ovarian cancer. Breast Cancer Res Treat 62:245
Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K (2002) Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol 20:2624
Elson CJ, Barker RN, Thompson SJ, Williams NA (1995) Immunologically ignorant autoreactive T cells, epitope spreading, and repertoire limitation. Immunol Today 16:71
Fisk B, Blevins TL, Wharton JT, Ioannides CG (1995) Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med 181:2109
Fisk B, Hudson JM, Kavanagh J, Wharton JT, Murray JL, Ioannides CG, Kudelka AP (1997) Existent proliferative responses of peripheral blood mononuclear cells from healthy donors and ovarian cancer patients to HER-2 peptides. Anticancer Res 17:45
Humphreys RE, Adams S, Koldzic G, Nedelescu B, Hofe E von, Xu M (2000) Increasing the potency of MHC class II-presented epitopes by linkage to Ii-Key peptide. Vaccine 18:2693
Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H (1998) The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 188:2357
Ioannides CG, Platsoucas CD, Freedman RS (1990) Immunological effects of tumor vaccines: II. T cell responses directed against cellular antigens in the viral oncolysates. In Vivo 4:17
Kallinteris NL, Hu H, Li Y, Lu X, Wu S, Gulfo GV, Humphreys RE, Xu M (in press) Ii-key/ MHC class II epitope hybrid peptide vaccines for HIV. Vaccine 21:4128
Knutson KL, Disis ML (2002) Clonal diversity of the T-cell population responding to a dominant HLA-A2 epitope of HER-2/neu after active immunization in an ovarian cancer patient. Hum Immunol 63:547
Knutson KL, Schiffman K, Disis ML (2001) Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest 107:477
Knutson KL, Schiffman K, Cheever MA, Disis ML (2002) Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide: results in short-lived peptide-specific immunity. Clin Cancer Res 8:1014
Kobayashi H, Wood M, Song Y, Appella E, Celis E (2000) Defining promiscuous MHC class II helper T-cell epitopes for the HER2/neu tumor antigen. Cancer Res 60:5228
Kuerer HM, Peoples GE, Sahin AA, Murray JL, Singletary SE, Castilleja A, Hunt KK, Gershenson DM, Ioannides CG (2002) Axillary lymph node cellular immune response to HER-2/neu peptides in patients with carcinoma of the breast. J Interferon Cytokine Res 22:583
Lee TV, Johnston DA, Thomakos N, Honda T, Efferson CL, Ioannides CG (2002) Helper peptide G89 (HER-2:777–789) and G89-activated cells regulate the survival of effectors induced by the CTL epitope E75 (HER-2, 369–377). Correlation with the IFN-gamma: IL-10 balance. Anticancer Res 22:1481
Lewis JJ, Janetzki S, Schaed S, Panageas KS, Wang S, Williams L, Meyers M, Butterworth L, Livingston PO, Chapman PB, Houghton AN (2000) Evaluation of CD8(+) T-cell frequencies by the Elispot assay in healthy individuals and in patients with metastatic melanoma immunized with tyrosinase peptide. Int J Cancer 87:391
Masood S, Bui MM (2000) Assessment of HER-2/neu over expression in primary breast cancers and their metastatic lesions: an immunohistochemical study. Ann Clin Lab Sci 30:259
McNeel DG, Nguyen LD, Storer BE, Vessella R, Lange PH, Disis ML (2000) Antibody immunity to prostate-cancer-associated antigens can be detected in the serum of patients with prostate cancer. J Urol 164:1825
Moudgil KD, Sercarz EE (2000) The self-directed T cell repertoire: its creation and activation. Rev Immunogenet 2:26
Murray JL, Gillogly ME, Przepiorka D, Brewer H, Ibrahim NK, Booser DJ, Hortobagyi GN, Kudelka AP, Grabstein KH, Cheever MA, Ioannides CG (2002) Toxicity, immunogenicity, and induction of E75-specific tumor-lytic CTLs by HER-2 peptide E75 (369–377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+ patients with metastatic breast and ovarian cancer. Clin Cancer Res 8:3407
Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ (1998) Phase-II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 16:2659
Reilly RT, Machiels JP, Emens LA, Ercolini AM, Okoye FI, Lei RY, Weintraub D, Jaffee EM (2001) The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res 61:880
Rongcun Y, Salazar-Onfray F, Charo J, Malmberg KJ, Evrin K, Maes H, Kono K, Hising C, Petersson M, Larsson O, Lan L, Appella E, Sette A, Celis E, Kiessling R (1999) Identification of new HER2/neu-derived peptide epitopes that can elicit specific CTL against autologous and allogeneic carcinomas and melanomas. J Immunol 163:1037
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783
Sotiriadou R, Perez SA, Gritzapis AD, Sotiropoulou PA, Echner H, Heinzel S, Mamalaki A, Pawelec G, Voelter W, Baxevanis CN, Papamichail M (2001) Peptide HER2 (776–788) represents a naturally processed broad MHC class II-restricted T cell epitope. Br J Cancer 85:1527
Tuttle TM, Anderson BW, Thompson WE, Lee JE, Sahin A, Smith TL, Grabstein KH, Wharton JT, Ioannides CG, Murray JL (1998) Proliferative and cytokine responses to class II HER-2/neu-associated peptides in breast cancer patients. Clin Cancer Res 4:2015
Ward RL, Hawkins NJ, Coomber D, Disis ML (1999) Antibody immunity to the HER-2/neu oncogenic protein in patients with colorectal cancer. Hum Immunol 60:510
Xu M, Jackson R, Adams S, Humphreys RE (1999) Studies on activities of invariant chain peptides on releasing or exchanging of antigenic peptides at human leukocyte antigen-DR1. Arzneimittelforschung 49:791
Zaks TZ, Rosenberg SA (1998) Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res 58:4902
Acknowledgement
The authors gratefully acknowledge the advice of C. Ioannides in performing the analyses and interpreting the results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gillogly, M.E., Kallinteris, N.L., Xu, M. et al. Ii-Key/HER-2/neu MHC class-II antigenic epitope vaccine peptide for breast cancer. Cancer Immunol Immunother 53, 490–496 (2004). https://doi.org/10.1007/s00262-003-0463-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-003-0463-y