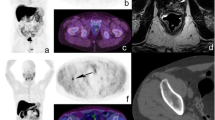
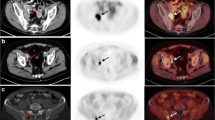
Abstract
Purpose
To evaluate the therapeutic impact of 18F-fluorocholine (FCH) PET/CT in biochemical recurrent prostate cancer (PC) and to investigate the value of quantitative FCH PET/CT parameters in predicting progression-free survival (PFS).
Methods
This retrospective study included 172 consecutive patients with PC who underwent FCH PET/CT for biochemical recurrence. Mean rising PSA was 10.7 ± 35.0 ng/ml. Patients with positive FCH PET were classified into three groups: those with uptake only in the prostatic bed, those with locoregional disease, and those with distant metastases. Referring physicians were asked to indicate the hypothetical therapeutic strategy with and without the FCH PET/CT results. Clinical variables and PET parameters including SUVmax, SUVpeak, SUVmean, total lesion choline kinase activity (TLCKA) and standardized added metabolic activity (SAM) were recorded and a multivariate analysis was performed to determine the factors independently predicting PFS.
Results
In 137 of the 172 patients, the FCH PET/CT scan was positive, and of these, 29.9 % (41/137) had prostatic recurrence, 42.3 % (58/137) had pelvic lymph node recurrence with or without prostatic recurrence, and 27.7 % (38/137) had distant metastases. The FCH PET/CT result led to a change in treatment plan in 43.6 % (75/172) of the 172 patients. Treatment was changed in 49.6 % (68/137) of those with a positive FCH PET/CT scan and in 20 % (7/35) of those with a negative FCH PET/CT scan. After a median follow-up of 29.3 months (95 % CI 18.9 – 45.9 months), according to multivariate analysis age <70 years, SAM ≥23 and SUVmean ≥3 were parameters independently predicting PFS. A nomogram constructed using the three parameters showed 49 months of PFS in patients with the best scores (0 or 1) and only 11 months in patients with a poor score (score 3).
Conclusion
This study indicates that a positive FCH PET result in PC patients with biochemical recurrence predicts a shorter PFS and confirms the major impact of the FCH PET result on the management of biochemical recurrent PC.





Similar content being viewed by others
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Malvezzi M, Bertuccio P, Levi F, Vecchia CL, Negri E. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24(3):792–800.
Soyka JD, Muster MA, Schmid DT, Seifert B, Schick U, Miralbell R, et al. Clinical impact of 18F-choline PET/CT in patients with recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2012;39(6):936–43.
Picchio M, Briganti A, Fanti S, Heidenreich A, Krause BJ, Messa C, et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Urol. 2011;59(1):51–60.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–79.
Punnen S, Cooperberg MR, D’Amico AV, Karakiewicz PI, Moul JW, Scher HI, et al. Management of biochemical recurrence after primary treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2013;64(6):905–15.
Kane CJ, Amling CL, Johnstone PAS, Pak N, Lance RS, Thrasher JB, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61(3):607–11.
Sciarra A, Panebianco V, Salciccia S, Cattarino S, Lisi D, Gentilucci A, et al. Modern role of magnetic resonance and spectroscopy in the imaging of prostate cancer. Urol Oncol. 2009;29(1):12–20.
Giovacchini G, Picchio M, Coradeschi E, Bettinardi V, Gianolli L, Scattoni V, et al. Predictive factors of [11C] choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2009;37:301–9.
Castellucci P, Fuccio C, Nanni C, Santi I, Rizzello A, Lodi F, et al. Influence of trigger PSA and PSA kinetics on 11C-Choline PET/CT detection rate in patients with biochemical relapse after radical prostatectomy. J Nucl Med. 2009;50(9):1394–400.
Kwee SA, Coel MN, Lim J. Detection of recurrent prostate cancer with 18F-fluorocholine PET/CT in relation to PSA level at the time of imaging. Ann Nucl Med. 2012;26(6):501–7.
Marzola MC, Chondrogiannis S, Ferretti A, Grassetto G, Rampin L, Massaro A, et al. Role of 18F-choline PET/CT in biochemically relapsed prostate cancer after radical prostatectomy: correlation with trigger PSA, PSA velocity, PSA doubling time, and metastatic distribution. Clin Nucl Med. 2013;38(1):e26–32.
Giovacchini G, Picchio M, Parra RG, Briganti A, Gianolli L, Montorsi F, et al. Prostate-specific antigen velocity versus prostate-specific antigen doubling time for prediction of 11C choline PET/CT in prostate cancer patients with biochemical failure after radical prostatectomy. Clin Nucl Med. 2012;37(4):325–31.
Beheshti M, Haim S, Zakavi R, Steinmair M, Waldenberger P, Kunit T, et al. Impact of 18F-choline PET/CT in prostate cancer patients with biochemical recurrence: influence of androgen deprivation therapy and correlation with PSA kinetics. J Nucl Med. 2013;54(6):833–40.
Detti B, Scoccianti S, Franceschini D, Cipressi S, Cassani S, Villari D, et al. Predictive factors of [18F]-Choline PET/CT in 170 patients with increasing PSA after primary radical treatment. J Cancer Res Clin Oncol. 2013;139(3):521–8.
Rybalov M, Breeuwsma AJ, Leliveld AM, Pruim J, Dierckx RA, de Jong IJ. Impact of total PSA, PSA doubling time and PSA velocity on detection rates of 11C-Choline positron emission tomography in recurrent prostate cancer. World J Urol. 2013;31(2):319–23.
Castellucci P, Fuccio C, Rubello D, Schiavina R, Santi I, Nanni C, et al. Is there a role for 11C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5 ng/ml? Eur J Nucl Med Mol Imaging. 2011;38(1):55–63.
Graute V, Jansen N, Ubleis C, Seitz M, Hartenbach M, Scherr MK, et al. Relationship between PSA kinetics and [18F] fluorocholine PET/CT detection rates of recurrence in patients with prostate cancer after total prostatectomy. Eur J Nucl Med Mol Imaging. 2012;39(2):271–82.
Memorial Sloan-Kettering Cancer Center. Prostate Cancer Nomograms: PSA Doubling Time. New York, NY: Memorial Sloan-Kettering Cancer Center, 2014. http://nomograms.mskcc.org/Prostate/PsaDoublingTime.aspx.
Vassiliev D, Krasikova R, Kutznetsova O, Federova O, Nader M. Simple HPLC method for the detection of N,N-dimethylaminoethanol in the preparation of [N-methyl-11C] choline. Eur J Nucl Med Mol Imaging. 2003;30(2 Suppl):342P.
Schmid DT, John H, Zweifel R, Cservenyak T, Westera G, Goerres GW, et al. Fluorocholine PET/CT in patients with prostate cancer: initial experience. Radiology. 2005;235(2):623–8.
Oprea-Lager DE, Vincent AD, van Moorselaar RJA, Gerritsen WR, van den Eertwegh AJM, Eriksson J, et al. Dual-phase PET-CT to differentiate [18F] Fluoromethylcholine uptake in reactive and malignant lymph nodes in patients with prostate cancer. PLoS One. 2012;7(10):e48430.
Mertens J, Dobbeleir A, Ham H, D’Asseler Y, Goethals I, Van de Wiele C. Standardized added metabolic activity (SAM): a partial volume independent marker of total lesion glycolysis in liver metastases. Eur J Nucl Med Mol Imaging. 2012;39(9):1441–8.
Brogsitter C, Zöphel K, Kotzerke J. 18F-Choline, 11C-choline and 11C-acetate PET/CT: comparative analysis for imaging prostate cancer patients. Eur J Nucl Med Mol Imaging. 2013;40 Suppl 1:S18–27.
FDA approves 11C-choline for PET in prostate cancer. J Nucl Med. 2012;53(12):11N.
Brenot-Rossi I. Focus: Prostate cancer and PET-choline. Prog Urol. 2014;24(1):3–8.
Ceci F, Herrmann K, Castellucci P, Graziani T, Bluemel C, Schiavina R, et al. Impact of (11)C-choline PET/CT on clinical decision making in recurrent prostate cancer: results from a retrospective two-centre trial. Eur J Nucl Med Mol Imaging. 2014;41(12):2222–31.
Giovacchini G, Picchio M, Garcia-Parra R, Briganti A, Abdollah F, Gianolli L, et al. 11C-choline PET/CT predicts prostate cancer-specific survival in patients with biochemical failure during androgen-deprivation therapy. J Nucl Med. 2014;55(2):233–41.
Giovacchini G, Incerti E, Mapelli P, Kirienko M, Briganti A, Gandaglia G, et al. [11C]Choline PET/CT predicts survival in hormone-naive prostate cancer patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2015;42(6):877–84.
Breeuwsma AJ, Rybalov M, Leliveld AM, Pruim J, de Jong IJ. Correlation of [11C] choline PET-CT with time to treatment and disease-specific survival in men with recurrent prostate cancer after radical prostatectomy. Q J Nucl Med Mol Imaging. 2012;56(5):440–446.
Visvikis D, Hatt M, Tixier F, Cheze Le Rest C. The age of reason for FDG PET image-derived indices. Eur J Nucl Med Mol Imaging. 2012;39(11):1670–2.
Krak NC, Boellaard R, Hoekstra OS, Twisk JWR, Hoekstra CJ, Lammertsma AA. Effects of ROI definition and reconstruction method on quantitative outcome and applicability in a response monitoring trial. Eur J Nucl Med Mol Imaging. 2005;32(3):294–301.
Mertens J, De Bruyne S, Van Damme N, Smeets P, Ceelen W, Troisi R, et al. Standardized added metabolic activity (SAM) in 18F-FDG PET assessment of treatment response in colorectal liver metastases. Eur J Nucl Med Mol Imaging. 2013;40(8):1214–22.
Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2(3):159–71.
Fleming JS, Tossici-Bolt L, Guy M, Kemp P. Comment on Mertens et al.: Standardized added metabolic activity (SAM): a partial volume independent marker of total lesion glycolysis in liver metastases. Eur J Nucl Med Mol Imaging. 2013;40(5):788–9.
Mahmood U. 2014 SNMMI highlights lecture: oncology. J Nucl Med. 2014;55(11):9N–24N.
Compliance with ethical standards
ᅟ
Funding
This work was supported by grants from the French National Agency for Research called “Investissements d’Avenir”, Labex IRON no. ANR-11-LABX-0018-01 and Equipex ArronaxPlus no. ANR-11-EQPX-0004.
Conflicts of interest
None.
Informed consent
For this retrospective study, we obtained informed consent from all patients to allow the use of their clinical data for research purposes under a protocol approved in our institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colombié, M., Campion, L., Bailly, C. et al. Prognostic value of metabolic parameters and clinical impact of 18F-fluorocholine PET/CT in biochemical recurrent prostate cancer. Eur J Nucl Med Mol Imaging 42, 1784–1793 (2015). https://doi.org/10.1007/s00259-015-3123-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3123-5