Abstract
Purpose
The objective of this study was to assess the prognostic value of metabolic tumor burden on 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG) positron emission tomography (PET)/CT measured with metabolic tumor volume (MTV) and total lesion glycolysis (TLG), independent of Union Internationale Contra la Cancrum (UICC)/American Joint Committee on Cancer (AJCC) tumor, node, and metastasis (TNM) stage, in comparison with that of standardized uptake value (SUV) in nonsurgical patients with non-small cell lung cancer (NSCLC).
Methods
This study retrospectively reviewed 169 consecutive nonsurgical patients (78 men, 91 women, median age of 68 years) with newly diagnosed NSCLC who had pretreatment 18F-FDG PET/CT scans. The 18F-FDG PET/CT scans were performed in accordance with National Cancer Institute guidelines. The MTV of whole-body tumor (MTVWB), of primary tumor (MTVT), of nodal metastases (MTVN), and of distant metastases (MTVM); the TLG of whole-body tumor (TLGWB), of primary tumor (TLGT), of nodal metastases (TLGN), and of distant metastases (TLGM); the SUVmax of whole-body tumor (SUVmaxWB), of primary tumor (SUVmaxT), of nodal metastases (SUVmaxN), and of distant metastases (SUVmaxM) as well as the SUVmean of whole-body tumor (SUVmeanWB), of primary tumor (SUVmeanT), of nodal metastases (SUVmeanN), and of distant metastases (SUVmeanM) were measured with the PETedge tool on a MIMvista workstation with manual adjustment. The median follow-up among survivors was 35 months from the PET/CT (range 2–82 months). Statistical methods included Kaplan-Meier curves, Cox regression, and C-statistics.
Results
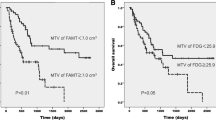
There were a total of 139 deaths during follow-up. Median overall survival (OS) was 10.9 months [95% confidence interval (CI) 9.0–13.2 months]. The MTV was statistically associated with OS. The hazard ratios (HR) for 1 unit increase of ln(MTVWB), √(MTVT), √(MTVN), and √(MTVM) before/after adjusting for stage were: 1.47/1.43 (p < 0.001/<0.001), 1.06/1.05 (p < 0.001/<0.001), 1.11/1.10 (p < 0.001/<0.001), and 1.04/1.03 (p = 0.007/0.043), respectively. TLG had statistically significant associations with OS with the HRs for 1 unit increase in ln(TLGWB), √(TLGT), √(TLGN), and √(TLGM) before/after adjusting for stage being 1.36/1.33 (p < 0.001/<0.001), 1.02/1.02 (p = 0.001/0.002), 1.05/1.04 (p < 0.001/<0.001), and 1.02/1.02 (p = 0.003/0.024), respectively. The ln(SUVmaxWB) and √(SUVmaxN) were statistically associated with OS with the corresponding HRs for a 1 unit increase before/after adjusting for stage being 1.46/1.43 (p = 0.013/0.024) and 1.22/1.16 (p = 0.002/0.040). The √(SUVmeanN) was statistically associated with OS before and after adjusting for stage with HRs for a 1 unit increase of 1.32 (p < 0.001) and 1.24 (p = 0.015), respectively. The √(SUVmeanM) and √(SUVmaxM) were statistically associated with OS before adjusting for stage with HRs for a 1 unit increase of 1.26 (p = 0.017) and 1.18 (p = 0.007), respectively, but not after adjusting for stage (p = 0.127 and 0.056). There was no statistically significant association between OS and √(SUVmaxT), ln(SUVmeanWB), or √(SUVmeanT). There was low interobserver variability among three radiologists with intraclass correlation coefficients (ICC) greater than 0.94 for SUVmaxWB, ln(MTVWB), and ln(TLGWB). Interobserver variability was higher for SUVmeanWB with an ICC of 0.806.
Conclusion
Baseline metabolic tumor burdens at the level of whole-body tumor, primary tumor, nodal metastasis, and distant metastasis as measured with MTV and TLG on FDG PET are prognostic measures independent of clinical stage with low inter-observer variability and may be used to further stratify nonsurgical patients with NSCLC. This study also suggests MTV and TLG are better prognostic measures than SUVmax and SUVmean. These results will need to be validated in larger cohorts in a prospective study.






Similar content being viewed by others
References
Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer 2001;37 Suppl 8:S4–S66.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010;60(5):277–300.
Traynor AM, Schiller JH. Systemic treatment of advanced non-small cell lung cancer. Drugs Today (Barc) 2004;40(8):697–710.
UyBico SJ, Wu CC, Suh RD, Le NH, Brown K, Krishnam MS. Lung cancer staging essentials: the new TNM staging system and potential imaging pitfalls. Radiographics 2010;30(5):1163–81.
American Joint Committee on Cancer. AJCC cancer staging manual. 6th ed. New York: Springer; 2002.
Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710–7.
Adebonojo SA, Bowser AN, Moritz DM, Corcoran PC. Impact of revised stage classification of lung cancer on survival: a military experience. Chest 1999;115:1507–13.
van Rens MT, de la Rivière AB, Elbers HR, van Den Bosch JM. Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest 2000;117:374–9.
Socinski MA, Morris DE, Masters GA, Lilenbaum R, American College of Chest Physicians. Chemotherapeutic management of stage IV non-small cell lung cancer. Chest 2003;123:226S–43S.
Lee P, Weerasuriya DK, Lavori PW, Quon A, Hara W, Maxim PG, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys 2007;69:328–33.
La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2009;74:1335–41.
Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging 1999;2:159–71.
Berkowitz A, Basua S, Srinivasa S, Sankarana S, Schuster S, Alavi A. Determination of whole-body metabolic burden as a quantitative measure of disease activity in lymphoma: a novel approach with fluorodeoxyglucose-PET. Nucl Med Commun 2008;29:521–6.
Roedl JB, Colen RR, Holalkere NS, Fischman AJ, Choi NC, Blake MA. Adenocarcinomas of the esophagus: response to chemoradiotherapy is associated with decrease of metabolic tumor volume as measured on PET-CT. Comparison to histopathologic and clinical response evaluation. Radiother Oncol 2008;89:278–86.
Lee P, Bazan JG, Lavori PW, Weerasuriya DK, Quon A, Le QT, Wakelee HA, Graves EE, Loo BW Jr. Metabolic tumor volume is an independent prognostic factor in patients treated definitively for non-small-cell lung cancer. Clin Lung Cancer 2011 Jun 22. [Epub ahead of print].
Zhu D, Ma T, Niu Z, Zheng J, Han A, Zhao S, et al. Prognostic significance of metabolic parameters measured by (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer 2011;73:332–7.
Seol YM, Kwon BR, Song MK, Choi YJ, Shin HJ, Chung JS, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with head and neck cancer treated by chemo-radiation therapy. Acta Oncol 2010;49:201–8.
Xie P, Yue JB, Zhao HX, Sun XD, Kong L, Fu Z, et al. Prognostic value of 18F-FDG PET-CT metabolic index for nasopharyngeal carcinoma. J Cancer Res Clin Oncol 2010;136(6):883–9.
Chung MK, Jeong H-S, Park SG, Jang JY, Son Y-I, Choi JY, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res 2009;15:5861–8.
Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol 2010;17:115–22.
Wang W, Larson SM, Fazzari M, Tickoo SK, Kolbert K, Sgouros G, et al. Prognostic value of [18F]fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab 2000;85:1107–13.
Bradley JD, Ieumwananonthachai N, Purdy JA, Wasserman TH, Lockett MA, Graham MV, et al. Gross tumor volume, critical prognostic factor in patients treated with three-dimensional conformal radiation therapy for non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 2002;52:49–57.
Doi K. Computer-aided diagnosis in medical imaging: historical review, current status and future potential. Comput Med Imaging Graph 2007;31:198–211.
Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AAA, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med 2006;47:1059–66.
Werner-Wasik M, Nelson AD, Choi W, Arai Y, Faulhaber PF, Kang P, Almeida FD, Xiao Y, Ohri N, Brockway KD, Piper JW, Nelson AS. What is the best way to contour lung tumors on PET scans? Multiobserver validation of a gradient-based method using a NSCLC digital PET phantom. Int J Radiat Oncol Biol Phys. 2011 Apr 28. [Epub ahead of print].
Cox DR. Regression models and life-tables (with discussion). J R Stat Soc Series B Stat Methodol 1972;34:187–220.
Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika 2005;92:965–70.
Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–26.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81.
Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86(2):420–8.
Borst GR, Belderbos JS, Boellaard R, Comans EF, De Jaeger K, Lammertsma AA, et al. Standardised FDG uptake: a prognostic factor for inoperable non-small cell lung cancer. Eur J Cancer 2005;41:1533–41.
Davies A, Tan C, Paschalides C, Barrington SF, O’Doherty M, Utley M, et al. FDG-PET maximum standardised uptake value is associated with variation in survival: analysis of 498 lung cancer patients. Lung Cancer 2007;55:75–8.
van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer 2007;43:1392–8.
de Geus-Oei LF, Oyen WJG. Predictive and prognostic value of FDG-PET. Cancer Imaging 2008;8:70–80.
William Jr WN, Lin HY, Lee JJ, Lippman SM, Roth JA, Kim ES. Revisiting stage IIIB and IV non-small cell lung cancer: analysis of the surveillance, epidemiology, and end results data. Chest 2009;136:701–9.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liao, S., Penney, B.C., Wroblewski, K. et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 39, 27–38 (2012). https://doi.org/10.1007/s00259-011-1934-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1934-6