Abstract
Background
Bone metastases are responsible for most of the morbidity associated with hormone-refractory prostate cancer (HRPC). 153Sm-ethylenediaminetetramethylene phosphonate (153Sm-EDTMP) has been approved for palliation of painful skeletal metastases. We retrospectively investigated the possible synergistic effect on survival of 153Sm-EDTMP (given to HRPC patients for bone pain palliation) and chemotherapy.
Methods
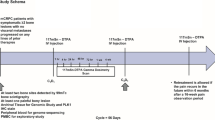
Forty-five HRPC patients were evaluated, with a median age of 71 years. The number of metastatic bone sites was ≤10 in 25 patients and >10 in 20 patients. Median serum PSA was 224 ng/ml. Bone pain was mild in 6 patients, moderate in 16, severe in 22 and intolerable in 1. Fifteen patients were only treated with 153Sm-EDTMP (group A), while 30 patients also received chemotherapy (estramustine phosphate or mitoxantrone plus prednisone) at variable times: between 3 and 5 months after 153Sm-EDTMP (14 patients, group B) or within 1 month after 153Sm-EDTMP (16 patients, group C).
Results
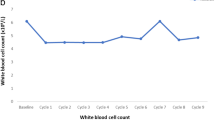
Haematological toxicities observed after either regimen were in general mild, consistent with common observations after either 153Sm-EDTMP or chemotherapy, and without any additive adverse effects in the patients receiving both 153Sm-EDTMP and chemotherapy. Bone pain palliation to some degree was induced by 153Sm-EDTMP in 32/45 patients (71.1%), the proportion of patients with a favourable clinical response being significantly higher in group C than in group A (87.5% vs 53.3%, p = 0.0388). Also in terms of biochemical response (serum PSA levels), patients of group C performed significantly better than patients of group A (p = 0.0235). Overall median survival from the time of administration of 153Sm-EDTMP was 15 months in the total cohort of 45 patients, and was significantly longer in group C than in either group B (30 months vs 11 months, p = 0.023) or group A (30 months vs 10 months, p = 0.008).
Conclusion
The results of this study confirm that 153Sm-EDTMP is effective in terms of pain relief and PSA response, with minimal toxicity. When it was administered in combination with chemotherapy, prolonged survival indicated actual clinical benefit, while there were no additive toxicities. These results provide the rationale for future prospective evaluation of combined therapeutic strategies.

Similar content being viewed by others
References
Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin 2003;53:5–26.
McRea LE, Karafin L. Carcinoma of the prostate: metastases, therapy, and survival—A statistical analysis of 500 cases. Int Coll Surg J 1958;29:723–8.
Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996;14:1756–64.
Sabbatini P, Larson SM, Kremer A, Zhang ZF, Sun M, Yeung H, et al. Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J Clin Oncol 1999;17:948–57.
Carroll PR, Kantoff PW, Balk SP, Brown MA, D’amico AV, George DJ, et al. Overview consensus statement. Newer approaches to androgen deprivation therapy in prostate cancer. Urology 2002;60 suppl 1:1–6.
Chodak GW, Keane T, Klotz L. Critical evaluation of hormonal therapy for carcinoma of the prostate. Urology 2002;60:201–8.
Tannock IF. Is there evidence that chemotherapy is of benefit to patients with carcinoma of the prostate? J Clin Oncol 1985;3:1013–21.
Eisenberger MA, Simon R, O’Dwyer PJ, Wittes RE, Friedman MA. A reevaluation of nonhormonal cytotoxic chemotherapy in treatment of prostatic carcinoma. J Clin Oncol 1985;3:827–41.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502–12.
Petrylak DP, Tangen CM, Hussain MHA, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513–20.
Serafini AN. Therapy of metastic bone pain. J Nucl Med 2001;42:895–906.
Lewington VJ. Bone-seeking radionuclides for therapy. J Nucl Med 2005;46:38S–47S.
Pandit-Taskar N, Batraki M, Divgi R. Radiopharmaceutical therapy for palliation of bone pain from osseous metastases. J Nucl Med 2004;45:1358–65.
Dafermou A, Colamussi P, Giganti M, Cittanti C, Bestagno M, Piffanelli A. A multicentre observational study of radionuclide therapy in patients with painful bone metastases of prostate cancer. Eur J Nucl Med 2001;28:788–98.
Serafini AN. Systemic metabolic radiotherapy with samarium-153-EDTMP for the treatment of painful bone metastasis. Quart J Nucl Med 2001;45:91–9.
Serafini AN. Samarium Sm-153 lexidronam for the palliation of bone pain associated with metastasis. Cancer 2000;88 suppl:2934–9.
Serafini AN, Houston SJ, Resche I, Quick DP, Grund FM, Ell PJ, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double blind placebo-controlled clinical trial. J Clin Oncol 1998;16:1574–81.
Sartor O, Reid R, Hoskin PJ, Quick DP, Ell PJ, Coleman RE, et al. Samarium-153-lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 2004;63:940–5.
Resche I, Chatal JF, Pecking A, Ell P, Duchesne G, Rubens R, et al. A dose-controlled study of Sm-153-EDTMP in the treatment of patients with painful bone metastasis. Eur J Cancer 1997;33:1583–91.
Sinzinger H, Weiss K, Granegger S, Ofluogu S, Kratzik CH, Hajek CH. The Vienna protocol on repeated low dose 153Sm-EDTMP therapy includes pain palliation and lesion regression/stabilization (abstract). Eur J Nucl Med 2001;28:1198.
Riva P, Franceschi G, Riva N, Casi M, Santimaria M. Anti-tumor effectiveness of multiple courses of 153Sm-EDTMP in bone metastasis from different cancers (abstract). Eur J Nucl Med 2001;28:1054.
Akerley W, Butera J, Wehbe T, Noto R, Stein B, Safran H, et al. A multiinstitutional, concurrent chemoradiation trial of strontium-89 extramustine and vinblastine for hormone refractory prostate carcinoma involving bone. Cancer 2002;94:1654–60.
Sciuto R, Festa A, Rea S, Pasqualoni R, Bergomi S, Petrilli G, et al. Effects of low-dose cisplatin on 89Sr therapy for painful bone metastases from prostate cancer: a randomized clinical trial. J Nucl Med 2002;43:79–86.
Pagliaro LC, Delpassand ES, Williams D, Millikan RE, Tu SM, Logothetis CJ. A phase I/II study of strontium-89 combined with gemcitabine in the treatment of patients with androgen independent prostate carcinoma and bone metastases. Cancer 2003;12:2988–94.
Logothetis C, Tu S, Navone N. Targeting prostate cancer bone metastases. Cancer 2003;97:785–8.
Valdés Olmos RA, Hoefnagel CA. Radionuclide therapy in oncology: the dawning of its concomitant use with other modalities? Eur J Nucl Med Mol Imaging 2004;31:929–31.
Tu S, Millikan RE, Mengistu B, Delpassand ES, Amato RJ, Pagliaro LC, et al. Bone-targeted therapy for advanced androgen independent carcinoma of the prostate: a randomised phase II trial. Lancet 2001;367:336–41
Palmedo H, Manka-Waluch A, Albers P, Schmidt-Wolf IG, Reinhardt M, Ezziddin S, et al. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: randomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidene-diphosphonate. J Clin Oncol 2003;21:2869–75.
Palmedo H, Bucerius J. Radionuclide therapy in oncology: repeated administrations of high dose rate radiopharmaceuticals (abstract). Eur J Nucl Med Mol Imaging 2004;31:1556.
Anderson P. Samarium for osteoblastic bone metastases and osteosarcoma. Expert Opin Pharmacother 2006;7:1475–86.
Anderson PM, Wiseman GA, Erlandson L, Rodriguez V, Trotz B, Dubansky SA, et al. Gemcitabine radiosensitization after high-dose samarium for osteoblastic osteosarcoma. Clin Cancer Res 2005;11:6895–900.
Rodriguez V, Erlandson L, Arndt CAS, Wiseman GA, Anderson PM. Low toxicity and efficacy of 153samarium-EDTMP and melphalan as a conditioning regimen for secondary acute myelogenous leukemia. Pediatr Transplantation 2005;9:122–6.
Knop S, Dohmer BM, Kanz L, Bares R, Einsele H. 153 Samarium-EDTMP in myeloablative dosage followed by a second autotransplantation in patients with relapsed multiple myeloma (abstract). Haematologica 2004;89:ECR36.
Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591–7.
Berry S, Waldron T, Winquist E, Lukka H. The use of bisphosphonates in men with hormone-refractory prostate cancer: a systematic review of randomized trials. Can J Urol 2006;13:3180–8.
Storto G, Klain M, Paone G, Liuzzi R, Molino L, Marinelli A, et al. Combined therapy with Sr-89 and zoledronic acid in patients with painful bone metastases. Bone 2006;39:35–41.
Laplanche A, Beuzeboc P, Lumbroso J, Di Palma M, Theodore C, Prapotnich D, et al. A phase II trial of docetaxel and samarium in patients with bone metastases from castration-refractory prostate cancer (CRPC) and a response or stabilization after induction docetaxel-estramustine (abstract). Proc Am Soc Clin Oncol 2006;24:4608.
Widmark A, Linne T, Modig H, Johansson L. Optimizing the time of co-administration of docetaxel and samarium-153 for advanced androgen independent carcinoma of the prostate (abstract). Proc Am Soc Clin Oncol 2003;22:433.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ricci, S., Boni, G., Pastina, I. et al. Clinical benefit of bone-targeted radiometabolic therapy with 153Sm-EDTMP combined with chemotherapy in patients with metastatic hormone-refractory prostate cancer. Eur J Nucl Med Mol Imaging 34, 1023–1030 (2007). https://doi.org/10.1007/s00259-006-0343-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0343-8