Abstract
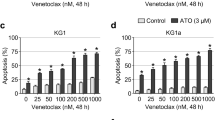
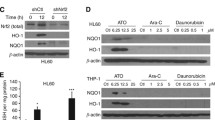
In the last decade, arsenic trioxide (As2O3) has been used very successfully to treat acute promyelocytic leukaemia (APL). Much less is known about the effectiveness of As2O3 in other neoplastic disorders. In this paper, we report that after 18 h in vitro treatment with 4 μM As2O3, 75 ± 18% of B cell chronic lymphocytic leukaemia (B-CLL) cells (n = 52) underwent apoptosis. It is important to note that B-CLL cells harboring a deletion of chromosome 17p13, which predisposes to fludarabine resistance and has been identified as an important negative predictor of clinical outcome, were more susceptible to As2O3 toxicity than cells lacking this aberration. Furthermore, unfavourable risk profiles such as unmutated IgVH status, high CD38 expression and prior treatment were associated with significantly higher sensitivity of B-CLL cells to As2O3. As2O3 also preferentially killed B-CLL cells compared to B cells from healthy age-matched controls. Molecular analysis revealed that basal superoxide dismutase activity was positively correlated with the pro-apoptotic activity of As2O3 pointing to a role of reactive oxygen species in cell death induction. The high activity of As2O3 in B-CLL cells from high-risk patients makes it a promising drug for high-risk and/or fludarabine-refractory B-CLL patients.






Similar content being viewed by others
References
Lanham S, Hamblin T, Oscier D, Ibbotson R et al (2003) Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood 101:1087–1093
Hamblin TJ, Orchard JA, Ibbotson RE, Davis Z et al (2002) CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood 99:1023–1029
Tinhofer I, Rubenzer G, Holler C, Hofstaetter E et al (2006) Expression levels of CD38 in T cells predict course of disease in male patients with B-chronic lymphocytic leukemia. Blood 108:2950–2956
Chen L, Widhopf G, Huynh L, Rassenti L et al (2002) Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood 100:4609–4614
Wiestner A, Rosenwald A, Barry TS, Wright G et al (2003) ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood 101:4944–4951
Stilgenbauer S, Kröber A, Busch R, Eichhorst B et al (2005) 17p deletion predicts for shorter overall survival after fludarabine-based first line therapy in chronic lymphocytic leukemia: First analysis of the CLL trial of the GCLLSG.. Onkologie 28(suppl 3):X–290:29–30
Sturm I, Bosanquet AG, Hermann S, Guner D et al (2003) Mutation of p53 and consecutive selective drug resistance in B-CLL occurs as a consequence of prior DNA-damaging chemotherapy. Cell Death Differ 10:477–484
Lozanski G, Heerema NA, Flinn IW, Smith L et al (2004) Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood 103:3278–3281
Byrd JC, Gribben JG, Peterson BL, Grever MR et al (2006) Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol 24:437–443
Eichhorst BF, Busch R, Hopfinger G, Pasold R et al (2006) Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood 107:885–891
Wierda W, O’Brien S, Wen S, Faderl S et al (2005) Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol 23:4070–4078
Hillmen P, Skotnicki A, Robak T, Jaksic B et al (2006) Alemtuzumab (CAMPATH(R), MABCAMPATH(R)) has superior progression free survival (PFS) vs chlorambucil as front-line therapy for patients with progressive B-cell chronic lymphocytic leukemia (BCLL). ASH Annual Meeting Abstracts 108:301
Byrd JC, Lin TS, Dalton JT, Wu D et al (2007) Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood 109:399–404
Raffoux E, Rousselot P, Poupon J, Daniel MT et al (2003) Combined treatment with arsenic trioxide and all-trans-retinoic acid in patients with relapsed acute promyelocytic leukemia. J Clin Oncol 21:2326–2334
Soignet SL, Maslak P, Wang ZG, Jhanwar S et al (1998) Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med 339:1341–1348
Shen ZY, Shen J, Cai WJ, Hong C et al (2000) The alteration of mitochondria is an early event of arsenic trioxide induced apoptosis in esophageal carcinoma cells. Int J Mol Med 5:155–158
Park WH, Seol JG, Kim ES, Hyun JM et al (2000) Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res 60:3065–3071
Zhou Y, Hileman EO, Plunkett W, Keating MJ et al (2003) Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood 101:4098–4104
Kitamura K, Minami Y, Yamamoto K, Akao Y et al (2000) Involvement of CD95-independent caspase 8 activation in arsenic trioxide-induced apoptosis. Leukemia 14:1743–1750
Scholz C, Richter A, Lehmann M, Schulze-Osthoff K et al (2005) Arsenic trioxide induces regulated, death receptor-independent cell death through a Bcl-2-controlled pathway. Oncogene 24:7031–7042
Scholz C, Wieder T, Starck L, Essmann F et al (2005) Arsenic trioxide triggers a regulated form of caspase-independent necrotic cell death via the mitochondrial death pathway. Oncogene 24:1904–1913
Liu Q, Hilsenbeck S, Gazitt Y (2003) Arsenic trioxide-induced apoptosis in myeloma cells: p53-dependent G1 or G2/M cell cycle arrest, activation of caspase-8 or caspase-9, and synergy with APO2/TRAIL. Blood 101:4078–4087
Cheson BD, Bennett JM, Grever M, Kay N et al (1996) National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 87:4990–4997
Crespo M, Bosch F, Villamor N, Bellosillo B et al (2003) ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med 348:1764–1775
Kulterer B, Friedl G, Jandrositz A, Sanchez-Cabo F et al (2007) Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genomics 8:70
Chen GQ, Shi XG, Tang W, Xiong SM et al (1997) Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood 89:3345–3353
el Rouby S, Thomas A, Costin D, Rosenberg CR et al (1993) p53 gene mutation in B-cell chronic lymphocytic leukemia is associated with drug resistance and is independent of MDR1/MDR3 gene expression. Blood 82:3452–3459
Pelicano H, Feng L, Zhou Y, Carew JS et al (2003) Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem 278:37832–37839
Bakan N, Taysi S, Yilmaz O, Bakan E et al (2003) Glutathione peroxidase, glutathione reductase, Cu-Zn superoxide dismutase activities, glutathione, nitric oxide, and malondialdehyde concentrations in serum of patients with chronic lymphocytic leukemia. Clin Chim Acta 338:143–149
Dai J, Weinberg RS, Waxman S, Jing Y (1999) Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood 93:268–277
Jing Y, Dai J, Chalmers-Redman RM, Tatton WG et al (1999) Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood 94:2102–2111
Huang P, Feng L, Oldham EA, Keating MJ et al (2000) Superoxide dismutase as a target for the selective killing of cancer cells. Nature 407:390–395
Carew JS, Nawrocki ST, Krupnik YV, Dunner K Jr. et al (2006) Targeting endoplasmic reticulum protein transport: a novel strategy to kill malignant B cells and overcome fludarabine resistance in CLL. Blood 107:222–231
Taylor BF, McNeely SC, Miller HL, Lehmann GM et al (2006) p53 suppression of arsenite-induced mitotic catastrophe is mediated by p21CIP1/WAF1. J Pharmacol Exp Ther 318:142–151
Damle RN, Temburni S, Calissano C, Yancopoulos S et al (2007) CD38 expression labels an activated subset within chronic lymphocytic leukemia clones enriched in proliferating B cells. Blood 110:3352–3359
Messmer BT, Messmer D, Allen SL, Kolitz JE et al (2005) In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest 115:755–764
Carew JS, Zhou Y, Albitar M, Carew JD et al (2003) Mitochondrial DNA mutations in primary leukemia cells after chemotherapy: clinical significance and therapeutic implications. Leukemia 17:1437–1447
Fayad L, Keating MJ, Reuben JM, O’Brien S et al (2001) Interleukin-6 and interleukin-10 levels in chronic lymphocytic leukemia: correlation with phenotypic characteristics and outcome. Blood 97:256–263
Ono M, Kohda H, Kawaguchi T, Ohhira M et al (1992) Induction of Mn-superoxide dismutase by tumor necrosis factor, interleukin-1 and interleukin-6 in human hepatoma cells. Biochem Biophys Res Commun 182:1100–1107
Dhar SK, Xu Y, Chen Y, St Clair DK (2006) Specificity protein 1-dependent p53-mediated suppression of human manganese superoxide dismutase gene expression. J Biol Chem 281:21698–21709
Acknowledgements
We thank Nathalie Wacht and Elisabeth Hofstätter for the excellent technical assistance in the preparation of PBMCs, data collection and FISH analysis. We are grateful for the very good collaboration with the laboratory of Dr. Hans Georg Mustafa. Finally, we want to thank Rajam Csordas for the critical reading of the manuscript. The support of the FWF-project P16153 to I.T., the SFB programme F021 to R.G. and I.T., the research grant from EBEWE, Austria and FFF project no. 810222, the Verein zur Förderung für Klinische Malignom und Zytokinforschung (Innsbruck–Salzburg), GEN-AU project GOLD and the research grant from the Jubiläumsfond der Österreichischen Nationalbank project no. 12170 to R.G. are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merkel, O., Heyder, C., Asslaber, D. et al. Arsenic trioxide induces apoptosis preferentially in B-CLL cells of patients with unfavourable prognostic factors including del17p13. J Mol Med 86, 541–552 (2008). https://doi.org/10.1007/s00109-008-0314-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-008-0314-6