Abstract
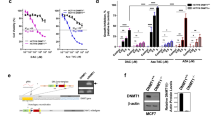
The deoxyribonucleic acid (DNA) methyltransferase (DNMT) inhibitor 5-aza-2′-deoxycytidine (5-aza-dC) has been used as a drug in a part of cancer therapy. However, because of its incorporation into DNA during DNA synthesis, 5-aza-dC can cause DNA damage, mutagenesis, and cytotoxicity. In view of the adverse effects of 5-aza-dC, DNMT-targeted inhibition may be a more effective approach than treatment with 5-aza-dC. To address the possibility of DNMT-targeted cancer therapy, we compared the effects of treatment with small interfering ribonucleic acids (siRNAs) specific for DNMT1 or DNMT3b and treatment with 5-aza-dC on transcription, cell growth, and DNA damage in gastric cancer cells. We found that DNMT1-targeted inhibition induced the re-expression and reversed DNA methylation of five (CDKN2A, RASSF1A, HTLF, RUNX3, and AKAP12B) out of seven genes examined, and 5-aza-dC reactivated and demethylated all seven genes. In contrast, DNMT3b siRNAs did not show any effect. Furthermore, the double knockdown of DNMT1 and DNMT3b did not show a synergistic effect on gene re-expression and demethylation. In addition, DNMT1 siRNAs showed an inhibitory effect of cell proliferation in the cancer cells and the induction of cell death without evidence of DNA damage, whereas treatment with 5-aza-dC caused DNA damage as demonstrated by the comet assay. These results provide a rationale for the development of a DNMT1-targeted strategy as an effective epigenetic cancer therapy.






Similar content being viewed by others
Abbreviations
- DNMT:
-
DNA methyltransferase
- 5-aza-dC:
-
5-aza-2′-deoxycytidine
- siRNA:
-
small interfering RNA
References
Jones PA, Baylin SB (2002) The fundamental role of epigenetic events in cancer. Nat Rev Genet 3:415–428
Rountree MR, Bachman KE, Herman JG, Baylin SB (2001) DNA methylation, chromatin inheritance, and cancer. Oncogene 20:3156–3165
Robertson KD (2001) DNA methylation, methyltransferases, and cancer. Oncogene 20:3139–3155
Rhee I, Jair KW, Yen RW, Lengauer C, Herman JG, Kinzler KW, Vogelstein B, Baylin SB, Schuebel KE (2000) CpG methylation is maintained in human cancer cells lacking DNMT1. Nature 404:1003–1007
Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE, Cui H, Feinberg AP, Lengauer C, Kinzler KW, Baylin SB, Vogelstein B (2002) DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature 416:552–556
Egger G, Jeong S, Escobar SG, Cortez CC, Li TW, Saito Y, Yoo CB, Jones PA, Liang G (2006) Identification of DNMT1 (DNA methyltransferase 1) hypomorphs in somatic knockouts suggests an essential role for DNMT1 in cell survival. Proc Natl Acad Sci USA 103:14080–14085
Robert MF, Morin S, Beaulieu N, Gauthier F, Chute IC, Barsalou A, MacLeod AR (2003) DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat Genet 33:61–65
Suzuki M, Sunaga N, Shames DS, Toyooka S, Gazdar AF, Minna JD (2004) RNA interference-mediated knockdown of DNA methyltransferase 1 leads to promoter demethylation and gene re-expression in human lung and breast cancer cells. Cancer Res 64:3137–3143
Ting AH, Jair KW, Suzuki H, Yen RW, Baylin SB, Schuebel KE (2004) CpG island hypermethylation is maintained in human colorectal cancer cells after RNAi-mediated depletion of DNMT1. Nat Genet 36:582–584
Beaulieu N, Morin S, Chute IC, Robert MF, Nguyen H, MacLeod AR (2002) An essential role for DNA methyltransferase DNMT3B in cancer cell survival. J Biol Chem 277:28176–28181
Leu YW, Rahmatpanah F, Shi H, Wei SH, Liu JC, Yan PS, Huang TH (2003) Double RNA interference of DNMT3b and DNMT1 enhances DNA demethylation and gene reactivation. Cancer Res 63:6110–6115
Juttermann R, Li E, Jaenisch R (1994) Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci USA 91:11797–11801
Zhu WG, Hileman T, Ke Y, Wang P, Lu S, Duan W, Dai Z, Tong T, Villalona-Calero MA, Plass C, Otterson GA (2004) 5-aza-2′-deoxycytidine activates the p53/p21Waf1/Cip1 pathway to inhibit cell proliferation. J Biol Chem 279:15161–15166
Weisenberger DJ, Velicescu M, Cheng JC, Gonzales FA, Liang G, Jones PA (2004) Role of the DNA methyltransferase variant DNMT3b3 in DNA methylation. Mol Cancer Res 2:62–72
Kang GH, Lee S, Kim JS, Jung HY (2003) Profile of aberrant CpG island methylation along multistep gastric carcinogenesis. Lab Invest 83:519–526
Kim TY, Jong HS, Jung Y, Kang GH, Bang YJ (2004) DNA hypermethylation in gastric cancer. Aliment Pharmacol Ther 20(Suppl 1):131–142
Kim TY, Jong HS, Song SH, Dimtchev A, Jeong SJ, Lee JW, Kim NK, Jung M, Bang YJ (2003) Transcriptional silencing of the DLC-1 tumor suppressor gene by epigenetic mechanism in gastric cancer cells. Oncogene 22:3943–3951
Choi MC, Jong HS, Kim TY, Song SH, Lee DS, Lee JW, Kim NK, Bang YJ (2004) AKAP12/Gravin is inactivated by epigenetic mechanism in human gastric carcinoma and shows growth suppressor activity. Oncogene 23:7095–7103
Kim TY, Lee HJ, Hwang KS, Lee M, Kim JW, Bang YJ, Kang GH (2004) Methylation of RUNX3 in various types of human cancers and premalignant stages of gastric carcinoma. Lab Invest 84:479–484
Ku JL, Park JG (2005) Biology of SNU cell lines. Cancer Res Treat 37:1–19
Robertson KD, Uzvolgyi E, Liang G, Talmadge C, Sumegi J, Gonzales FA, Jones PA (1999) The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic Acids Res 27:2291–2298
Dammann R, Li C, Yoon JH, Chin PL, Bates S, Pfeifer GP (2000) Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet 25:315–319
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Olive PL, Durand RE (2005) Heterogeneity in DNA damage using the comet assay. Cytometry A 66:1–8
Ting AH, Jair KW, Schuebel KE, Baylin SB (2006) Differential requirement for DNA methyltransferase 1 in maintaining human cancer cell gene promoter hypermethylation. Cancer Res 66:729–735
Chen T, Tsujimoto N, Li E (2004) The PWWP domain of Dnmt3a and Dnmt3b is required for directing DNA methylation to the major satellite repeats at pericentric heterochromatin. Mol Cell Biol 24:9048–9058
Gius D, Cui H, Bradbury CM, Cook J, Smart DK, Zhao S, Young L, Brandenburg SA, Hu Y, Bisht KS, Ho AS, Mattson D, Sun L, Munson PJ, Chuang EY, Mitchell JB, Feinberg AP (2004) Distinct effects on gene expression of chemical and genetic manipulation of the cancer epigenome revealed by a multimodality approach. Cancer Cell 6:361–371
Santini V, Kantarjian HM, Issa JP (2001) Changes in DNA methylation in neoplasia: pathophysiology and therapeutic implications. Ann Intern Med 134:573–586
Leone G, Voso MT, Teofili L, Lubbert M (2003) Inhibitors of DNA methylation in the treatment of hematological malignancies and MDS. Clin Immunol 109:89–102
Yang AS, Doshi KD, Choi SW, Mason JB, Mannari RK, Gharybian V, Luna R, Rashid A, Shen L, Estecio MR, Kantarjian HM, Garcia-Manero G, Issa JP (2006) DNA methylation changes after 5-aza-2′-deoxycytidine therapy in patients with leukemia. Cancer Res 66:5495–5503
Brueckner B, Boy RG, Siedlecki P, Musch T, Kliem HC, Zielenkiewicz P, Suhai S, Wiessler M, Lyko F (2005) Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases. Cancer Res 65:6305–6311
Stresemann C, Brueckner B, Musch T, Stopper H, Lyko F (2006) Functional diversity of DNA methyltransferase inhibitors in human cancer cell lines. Cancer Res 66:2794–2800
Szyf M (2005) DNA methylation and demethylation as targets for anticancer therapy. Biochemistry (Mosc) 70:533–549
Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP (2004) Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432:173–178
Behlke MA (2006) Progress towards in vivo use of siRNAs. Mol Ther 13:644–670
Schiffelers RM, Ansari A, Xu J, Zhou Q, Tang Q, Storm G, Molema G, Lu PY, Scaria PV, Woodle MC (2004) Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucleic Acids Res 32:e149
Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A (2005) RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther 12:461–466
Acknowledgments
This work was supported in part by grants from the Korean Ministry of Science and Technology through the National Research Laboratory Program for Cancer Epigenetics (No. M10400000336-06J0000-33610), and BK21 Project for Medicine, Dentistry and Pharmacy.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Primer sequences for RT-PCR analysis (PDF 180 KB)
Supplementary Table 2
Primer sequences for MS-PCR analysis (PDF 130 KB)
Supplementary Figure 1
(PDF 96.1 KB)
Supplementary Figure 2
(PDF 22.8 KB)
Rights and permissions
About this article
Cite this article
Jung, Y., Park, J., Kim, T.Y. et al. Potential advantages of DNA methyltransferase 1 (DNMT1)-targeted inhibition for cancer therapy. J Mol Med 85, 1137–1148 (2007). https://doi.org/10.1007/s00109-007-0216-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-007-0216-z