Abstract
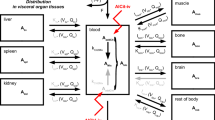
The pharmacokinetics of boric acid (BA) have been studied in animals and humans. Orally administered BA is readily and completely absorbed in rats, rabbits, and humans, as well as other animal species. In animals and humans, absorbed BA appears to be rapidly distributed throughout the body water via passive diffusion. Following administration of BA, the ratio of blood : soft tissue concentrations of boron (B) is approx 1.0 in rats and humans; in contrast, concentrations of B in bone exceed those in blood by a factor of approx 4 in both rats and humans. In rats, adipose tissue concentrations of B are only 20% of the levels found in blood and soft tissues; however, human data on adipose tissue levels are not available. BA does not appear to be metabolized in either animals or humans owing to the excessive energy required to break the B-O bond. BA has an affinity forcis-hydroxy groups, and it has been hypothesized to elicit its biological activity through this mechanism.
The elimination kinetics of BA also appear to be similar for rodents and humans. BA is eliminated unchanged in the urine. The kinetics of elimination were evaluated in human volunteers given BA orally or intravenously; the half-life for elimination was essentially the same (approx 21 h) by either route of exposure. In rats, blood and tissue levels of B reached steady-state after 3–4 d of oral administration of BA; assuming first-order kinetics, a half-life of 14–19 h may be calculated. The lack of metabolism of BA eliminates metabolic clearance as a potential source of interspecies variation. Accordingly, in the absence of differences in metabolic clearance, renal clearance is expected to be the major determinant of interspecies variation in pharmacokinetics. Because glomerular filtration rates are slightly higher in rats than in humans, the slight difference in half-lives may be readily explained.
The most sensitive toxicity end point for BA appears to be developmental toxicity in rats, with a No Observed Adverse Effect Level (NOAEL) and Lowest Observed Adverse Effect Level (LOAEL) of 55 and 76 mg BA/kg/d, respectively. Mean blood B levels in pregnant rats on gestation day 20 in the pivotal developmental toxicity study were reported to be 1.27 and 1.53 mcg B/g at the NOAEL and LOAEL, respectively. Blood B concentrations in humans are well below these levels. Average blood B levels in the most heavily exposed worker population at a borate mine was 0.24 mcg B/mL, and the estimated daily occupational exposure was equivalent to 160 mg BA/d. Blood B levels in the general population generally range from 0.03 to 0.09 mcg B/mL. These blood B values indicate an ample margin of safety for humans.
In summary, the pharmacokinetics of BA in humans and rodents are remarkably similar, and interspecies differences in pharmacokinetics appear to be minimal.
Similar content being viewed by others
References
W. G. Woods, An introduction to boron: history, sources, uses and chemistry,Environ. Health Perspect. 102(7), 5–11 (1994).
D. J. Fort, Adverse reproductive and developmental effects inXenopus from insufficient boron,Biol. Trace Element Res. (current vol).
C. D. Eckert, Essentiality of boron for vertebrate embryonic development in zebrafish and trout,Biol. Trace Element Res. (current vol.).
C. L. Keen, Effects of very low boron exposure on rat development,Biol. Trace Element Res. (current vol.).
F. H. Nielsen, The saga of boron in food: from a banished food preservative to a beneficial nutrient for humans,Curr. Topics Plant Biochem. Physiol. 10, 274 (1991).
F. H. Nielsen, Facts and fallacies about boron,Nutr. Today 27(3), 6–12 (1992).
F. H. Nielsen, Biochemical and physiologic consequences of boron deprivation in humans,Environ. Health Perspectives 102(7), 59–63 (1994).
W. Mertz, Essential trace metals: New definitions based on new paradigms,Nutr. Rev. 51(10), 287–295 (1993).
C. D. Hunt, The biochemical effects of physiologic amounts of dietary boron in animal nutrition models,Environ. Health Perspectives 102(7), 35–43 (1994).
C. D. Hunt, J. L. Herbel, and J. P. Idso, Dietary boron modifies the effects of vitamin D3 nutrition on indices of energy substrate utilization and mineral metabolism in the chick,J. Bone Miner. Res. 9(2), 171–182 (1994).
C. D. Hunt and B. J. Stoecker, Deliberations and evaluations of the approaches, end-points, and paradigms for boron, chromium, and fluoride dietary recommendations,J. Nutr. 126, 2441–2451 (1996).
C. D. Hunt, J. L. Herbel, and F. H. Nielsen, Metabolic response of postmenopausal women to supplemental dietary boron and aluminum during usual and low magnesium intake: boron, calcium, and magnesium absorption and retention and blood mineral concentrations,Am. J. Clin. Nutr. 65, 803–813 (1997).
C. J. Rainey, R. E. Christensen, L. A. Nyquist, P. L. Strong, and J. R. Coughlin, Boron daily intake from the American diet,FASEB J. 10, A785 (abstract no. 4536) (1996).
Environmental Protection Agency, Health and Environmental Effects Document for Boron and Boron Compounds, Environmental Criteria and Assessment Office, Cincinnati, Report EPA/600/8-91/015; Order No. PB91-233635, pp. 1–269 (1991).
ATSDR, Toxicological profile for boron, U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, pp. 1–86 (1992).
B. D. Culver, R. G. Smith, R. J. Brotherton, P. L. Strong, and T. M. Gray, Boron, inIndustrial Hygiene and Toxicology, 4th ed., vol. 2F, Patty’s, ed., John Wiley, New York 4411–1448 (1994).
F. J. Murray, A human health risk assessment of boron (boric acid and borax) in drinking water,Regul. Toxicol. Pharmacol. 22, 221–230 (1995).
Institute for Evaluating Health Risks, An assessment of boric acid and borax using the IEHR evaluative process for assessing human developmental and reproductive toxicity of agents, NTIS Technical Report PB96-156005 (1995).
European Centre for Ecotoxicology and Toxicology of Chemicals, Reproductive and General Toxicology of some Inorganic Borates and Risk Assessment for Human Beings, Technical Report No. 63, Brussels (1995).
S. A. Hubbard, Comparative toxicology of borates,Biol. Trace Element Res. (current vol.).
F. J. Murray, Issues in boron risk assessment: pivotal study, uncertainty factors, and ADIs,J. Trace Element Exp. Med. 9, 231–243 (1996).
C. J. Price, P. L. Strong, M. C. Marr, C. B. Myers, and F. J. Murray, Developmental toxicity NOAEL and postnatal recovery in rats fed boric acid during gestation,Fundam. Appl. Toxicol. 32, 179–193 (1996).
J. S. Schou, J. A. Jansen, and B. Aggerbeck, Human pharmacokinetics and safety of boric acid,Arch. Toxicol. 7, 232–235 (1984).
J. A. Jansen, J. S. Schou, and B. Aggerbeck, Gastrointestinal absorption and in vitro release of boric acid from water-emulsifying ointments,Food Chem. Toxicol. 22, 49–53 (1984).
C. Job, Absorption and excretion of orally administered boron,Z. Angew. Bader-und Klimaheilkunde 20, 137–142 (1973).
W. W. Ku, R. E. Chapin, R. F. Moseman, R. E. Brink, K. D. Pierce, and K. Y. Adams, Tissue disposition of boron in male Fischer rats,Toxicol. Appl. Pharmacol. 111, 145–151 (1991).
J. H. Draize and E. A. Kelley, The urinary excretion of boric acid preparations following oral administration and topical applications to intact and damaged skin of rabbits,Toxicol. Appl. Pharmacol. 1, 267–276 (1959).
T. F. Brown, M. E. McCormick, D. R. Morris, and L. K. Zeringue, Effects of dietary boron on mineral balance in sheep,Nutr. Res. 9, 503–512 (1989).
E. C. Owen, The excretion of borate by the dairy cow,J. Dairy Res. 13, 243 (1944).
H. J. Weeth, C. F. Speth, and D. R. Hanks, Boron content of plasma and urine as indicators of boron intake in cattle,Am. J. Vet. Res. 42, 474–477 (1981).
H. I. Maibach, In vivo percutaneous absorption of boric acid, borax, and disodium octaborate tetrahydrate in humans,Biol. Trace Element Res. (current vol.).
B. Friis-Hansen, B. Aggerbeck, and J. A. Jansen, Unaffected blood boron levels in new-born infants treated with a boric acid ointment,Food Chem. Toxicol. 20, 451–454 (1982).
K. H. Beyer, W. F. Bergfeld, W. O. Berndt, R. K. Boutewell, W. W. Carlton, D. K. Hoffman, et al., Final report on the safety assessment of sodium borate and boric acid,J. Am. Coll. Toxicol. 2(7), 87–125 (1983).
G. Stuttgen, T. Siebel, and B. Aggerbeck, Absorption of boric acid through human skin depending on the type of vehicle,Arch. Dermatol. Res. 272, 21–29 (1982).
G. H. Nielsen, Percutaneous absorption of boric acid from boron-containing preparations in rats,Acta Pharmacol. Toxicol. 28, 413–424 (1970).
G. V. Alexander, R. E. Nusbaum, and N. S. MacDonald, The boron and lithium content of human bones,J. Biol. Chem. 192, 489–496 (1951).
R. M. Forbes, A. R. Cooper, and H. H. Mitchell, On the occurrence of beryllium, boron, cobalt, and mercury in human tissues,J. Biol. Chem. 209, 857–864 (1954).
N. L. Ward, The determination of boron in biological materials by neutron irradiation and prompt gamma-ray spectrometry,J. Radioanalytical Nuclear Chem. 110(2), 633–639 (1987).
J. A. Jansen, J. Andersen, and J. S. Schou, Boric acid single dose pharmacokinetics after intravenous administration to man,Arch. Toxicol. 55, 64–67 (1984).
B. D. Culver, P. T. Shen, T. H. Taylor, A. Lee-Feldstein, H. Anton-Culver, and P. L. Strong, The relationship of blood-and urine-boron to boron exposure in borax-workers and the usefulness of urine-boron as an exposure marker,Environ. Health Perspectives 102(7), 133–137 (1994).
R. M. Forbes and H. H. Mitchell, Accumulation of dietary boron and strontium in young and adult albino rats,Arch. Ind. Health 16, 489–492 (1957).
K. A. Treinen and R. E. Chapin, Development of testicular lesions in F344 rats after treatment with boric acid,Toxicol. Appl. Pharmacol. 107, 325–335 (1991).
W. W. Ku, R. E. Chapin, R. N. Wine, and B. C. Gladen, Testicular toxicity of boric acid (BA): Relationship of dose to lesion development and recovery in the F344 rat,Reprod. Toxicol. 7, 305–319 (1993).
H. R. Massie, V. R. Aiello, A. E. Shumway, and T. Armstrong, Calcium, iron, copper, boron, collagen and density changes in bone with aging in C57BL/65 mice,Exp. Gerontol. 25, 469–481 (1990).
M. Laurent-Pettersson, B. Delpech, and M Hellier, The mapping of natural boron in histological sections of mouse tissues by the use of neutron-capture radiography,Histochem. J. 24, 939–950 (1992).
J. Emsley,The Elements, Clarendon, Oxford, p. 32 (1989).
T. L. Litovitz, W. Klein-Schwartz, G. M. Oderda, and B. F. Schmitz, Clinical manifestations of toxicity in a series of 784 boric acid ingestions,Am. J. Emerg. Med. 6, 209–213 (1988).
L. E. Farr and T. Konikowski, The renal clearance of sodium pentaborate in mice and men,Clin. Chem. 9, 717–726 (1963).
W. B. Clarke, C. E. Weber, and M. Koekebakker, Lithium and boron in human blood,J. Lab. Clin. Med. 109, 155–158 (1987).
F. R. Abou-Shakra, J. M. Havercroft, and N. I. Ward, Lithium and boron in biological tissues and fluids,Trace Elements in Med. 6(4), 142–146 (1989).
H. R. Imbus, J. Cholak, L. H. Miller, and T. Sterling, Boron, cadmium, chromium, and nickel in blood and urine,Arch. Environ. Health 6, 286–295 (1963).
F. H. Nielsen, Dietary supplementation of physiological amounts of boron increases plasma and urinary boron of perimenopausal women,Proc. North Dakota Acad. Sci. 50, (1996).
C. J. Price, P. L. Strong, F. J. Murray, and M. M. Goldberg, Blood boron concentrations in pregnant rats fed boric acid throughout gestation,Reprod. Toxicol. 11(6), 833–842 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murray, F.J. A comparative review of the pharmacokinetics of boric acid in rodents and humans. Biol Trace Elem Res 66, 331–341 (1998). https://doi.org/10.1007/BF02783146
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02783146