Abstract
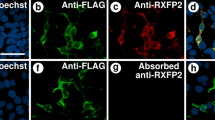
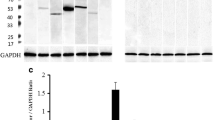
Vitamin D3, via its active metabolite 1,25-dihydroxyvitamin D3, plays a critical part in male and female reproduction in the rat. 1,25-Dihydroxyvitamin D3 activity is mediated by an intracellular receptor (VDR). VDR distribution in reproductive tissue has not been studied using antibodies against the receptor. We developed a polyclonal antibody against the VDR and used it to examine VDR distribution in male and female rat reproductive tissues. In rat testes, VDR epitopes were observed in seminiferous tubules, specifically in spermatogonia, Sertoli cells and spermatocytes. Spermatozoa stained faintly. Epithelial cells of the epididymis, seminal vesicles and prostate also expressed VDR epitopes. In the female rat reproductive tract, immunostaining for VDR was seen in ovarian follicles, specifically in granulosa cells. Weaker VDR immunostaining was observed in follicular thecal cells and in the ovarian stroma and germinal epithelium. Corpus luteal cells stained intensely for VDR. Epithelium of fallopian tubes and the uterus also contained VDR epitopes. Both nuclear and cytoplasmic VDR immunostaining was observed in male and female rat reproductive tissues. We conclude that the VDR is widely distributed in male and female reproductive tissues and that it is likely to mediate actions of 1,25-dihydroxyvitamin D3 in the tissues.
Similar content being viewed by others
References
Audran M, Gross M, Kumar R (1986) Physiology of the vitramin D endocrine system. Semin Nephrol 6:4–20
Dokoh S, Donaldson CA, Marion SL, Pike JW, Haussler MR (1983) The ovary: a target organ for 1,25-dihydroxyvitamin D3. Endocrinology 112:200–206
Erickson GF, Magoffin DA, Cragun JR, Chang RJ (1990) The effects of insulin and insulin-like growth factors-I and-II on estradiol production by granulosa cells of polycystic ovaries. J Clin Endocrinol Metab 70:894–902
Geneser F (1986) Textbook of histology. Munksgaard/Lea & Febiger, Philadelphia, Pa
Fujimoto G, Tsuchiva H, Kobayashi K, Saga M, Rothman CM, Ogawa S (1994) Calcium concentration and fertilization by subzonal insemination of a single spermatozoon in mouse oocytes. Mol Reprod Dev 38:54–60
Halloran BP, DeLuca HF (1980) Effect of vitamin D deficiency on fertility and reproductive capacity in the female rat. J Nutr 110:1573–1580
Henry HL, Dutta C, Cunningham, Blanchard R, Penny R, Tang C, Marchetto G, Chou Sy (1992) The cellular and molecular regulation of 1,25(OH)2D3 production. J Steroid Biochem Mol Biol 41:401–407
Hickie JP, Lavigne DM, Woodward WD (1983) Reduced fecundity of vitamin D deficient rats. Comp Biochem Physiol [A] 74:923–925
Hodgins MB, Murad S (1986) 1,25-Dihydroxycholecalciferol stimulates conversion of androstenedione into oestrone by human skin fibroblasts in culture. J Endocrinol 110:R1–4
Johnson JA, Kumar R (1994a) Renal and intestinal calcium transport: role of vitamin D and vitamin D-dependent calcium binding proteins. Semin Nephrol 14:119–128
Johnson JA, Kumar R (1994b) Vitamin D and renal calcium transport. Curr Opin Nephrol Hyperten 3:424–429
Kumar R, Schaefer J, Wieben E (1992) The expression of milligram amounts of functional human 1,25-dihydroxyvitamin D3 receptor in a bacterial expression system. Biochem Biophys Res Commun 189:1417–1423
Kumar R, Schaefer J, Grande JP, Roche PC (1994) Immuno-localization of the calcitriol receptor, 24-hydroxylase cytochrome P-450 and calbindin D28k in human kidney. Am J Physiol 266:F477-F485
Kwiecinski GG, Petrie GI, DeLuca HF (1989a) Vitamin D is necessary for reproductive functions of the male rat. J Nutr 119:741–744
Kwiecinski GG, Petrie GI, DeLuca HF (1989b) 1,25-Dihydroxyvitamin D3 restores fertility of vitamin D-deficient female rats. Am J Physiol 256:E483-E487
Levy FO, Eikvar L, Jutte NHPM, Cervenka J, Yoganathan T, Hansson V (1985a) Appearance of the rat testicular receptor for calcitriol (1,25-dihydroxyvitamin D3) during development. J Steroid Biochem 23:51–56
Levy FO, Eikvar L, Jutte NHPM, Froysa A, Tvermyr SM, Hansson V (1985b) Properties and compartmentalilization of the testicular receptor for 1,25-dihydroxyvitamin D3. J Steroid Biochem 22:453–460
Lieberherr M, Acker GM, Grosse B, Pesty A, Balsan S (1984) Rat endometrial cells in primary culture: effects and interaction of sex hormones and vitamin D3 metabolite on alkaline phosphatase. Endocrinology 115:824–829
Merke J, Hugel U, Ritz E (1985) Nuclear testicular 1,25-dihydroxyvitamin D3 receptors in sertoli cells and seminiferous tubules of adult rodents. Biochem Biophys Res Commun 127:303–309
Miller GJ, Stapleton GE, Ferrara JA, Lucia MS, Pfister S, Hedlund TE, Upadhya P (1992) The human prostatic carcinoma cell line LNCaP expresses biologically active, specific receptors for 1 alpha, 25-dihydroxyvitamin D3. Cancer Res 52:515–520
Narbaitz R, Tsang CP, Grunder AA (1987) Effects of vitamin D deficiency in the chicken embryo. Calcif Tissue Int 40:109–113
Osmundsen BC, Huang HF, Anderson MB, Christakos S, Walters MR (1989) Multiple sites of action of the vitamin D endocrine system. FSH stimulation of testis 1,25-dihydroxyvitamin D3 receptors. J Steroid Biochem 34:339–343
Peehl DM, Skowronski RJ, Leung GK, Wong ST, Stamey TA, Feldman D (1994) Antiproliferative effects of 1,25-dihydroxyvitamin D3 on primary cultures of human prostatic cells. Cancer Res 54:805–810
Potashnik G, Lunenfeld E, Levitas E, Itskovitz J, Albutiano S, Yankowitz N, Sonin Y, Levy J, Glezerman M, Shany S (1992) The relationship between endogenous oestradiol and vitamin D3 metabolites in serum and follicular fluid during ovarian stimulation for in-vitro fertilization and embryo transfer. Hum Reprod 7:1357–1360
Schleicher G, Privette TH, Stumpf WE (1989) Distribution of soltriol (1,25(OH)2D3) binding sites in male sex organs of the mouse: an autoradiographic study. J Histochem Cytochem 37:1083–1086
Schwartz GG, Hulka BS (1990) Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis). Anticancer Res 10:1307–1311
Skowronski RJ, Peehl DM, Feldman D (1993) Vitamin D and prostate cancer: 1,25-dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology 132:1952–1960
Sonnenberg J, Luine VN, Krey LC, Christakos S (1986) 1,25-Dihydroxyvitamin D3 treatment results in increased choline acetyltransferase activity in specific brain nuclei. Endocrinology 188:1433–1439
Stumpf WE (1988) Vitamin D-soltriol the heliogenic steroid hormone: somatotrophic activator and modulator. Discoveries from histochemical studies lead to new concepts. Histochemistry 89:209–219
Stumpf WE, Sar M, Chen K, Morin J, DeLuca HF (1987) Sertoli cells in the testis and epithelium of the ductuli efferentes are targets for3H 1,25(OH)2D3. An autoradiographic study. Cell Tissue Res 247:453–455
Stumpf WE, Denny ME (1989) Vitamin D (solitriol), light, and reproduction. Am J Obstet Gynecol 161:1375–1384
Walters MR (1981) An estrogen-stimulated 1,25-dihydroxyvitamin D3 receptor in rat uterus. Biochem Biophys Res Commun 103:721–726
Walters MR (1984) 1,25-Dihydroxyvitamin D3 receptors in the seminiferous tubules of the rat testis increase at puberty. Endocrinology 114:2167–2174
Walters MR (1992) Newly identified actions of the vitamin D endocrine system. Endocrine Rev 13:719–764
Walters MR, Cuneo DL, Jamison AP (1983) Possible significance of new target tissues for 1,25 dihydroxyvitamin D3. J Steroid Biochem 19:913–920
Zofkova I, Scholz G, Starka L (1989) Effect of calcitonin and 1,25(OH)2D3 on the FSH, LH and testosterone secretion at rest and LHRH stimulated secretion. Horm Metab Res 21:682–685
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, J.A., Grande, J.P., Roche, P.C. et al. Immunohistochemical detection and distribution of the 1,25-dihydroxyvitamin D3 receptor in rat reproductive tissues. Histochem Cell Biol 105, 7–15 (1996). https://doi.org/10.1007/BF01450873
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01450873