Summary
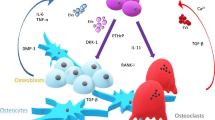
Metastasis of breast cancer cells to bone consists of multiple sequential steps. To accomplish the process of metastasis to bone, breast cancer cells are required to intrinsically possess or acquire the capacities that are necessary for them to proliferate, invade, migrate, survive, and ultimately arrest in bone. These capacities are essential for any cancer cells to develop distant metastases in organs such as lungs and liver as well as bone. Once breast cancer cells arrest in bone, bone is a storehouse of a variety of cytokines and growth factors and thus provides an extremely fertile environment for the cells to grow. However, breast cancer cells are unable to progress in bone unless they destroy bone with the assistance of bone-resorbing osteoclasts. Thus, the capacity of breast cancer cells to collaborate with osteoclasts is likely to be specific and is likely critical for them to cause osteolytic bone metastases. Evidence to support the concept that there is an intimate relationship between breast cancer cells and osteoclasts is described using anin vivo bone metastasis model in which human breast cancer cells are inoculated into the left ventricle of nude mice. The roles of cell adhesion molecules including cadherins and laminin and matrix metalloproteinases in the development of osteolytic bone metastases by breast cancer are also discussed.
Similar content being viewed by others
References
Walther HE: Krebsmetastasen. Bens Schwabe Verlag, Basel, Switzerland, 1948.
Paget S: The distribution of secondary growths in cancer of the breast. Lancet i:571–573, 1889.
Metcalf D: Hematopoietic regulators: Redundancy or subtlety. Blood 82:3515–3523, 1993.
Mundy GR, Roodman GD: Osteoclast ontogeny and function.In: Peck WA (ed) Bone and Mineral Research, V. Elsevier, 1987, pp 209–280.
Owen M, Friedenstein AJ: Stromal stem cells. Marrow-derived osteogenic precursors in cell and molecular biology of vertebrate hard tissues. CIBA Found Symp 136:41–60, 1988.
Orr W, Varani J, Gondek MD, Ward PA, Mundy GR: Chemotactic responses of tumor cells to products of resorbing bone. Science 203:176–177, 1979.
Mundy GR, DeMartina S, Rowe DW: Collagen and collagen fragments are chemotactic for tumor cells. J Clin Invest 68:1102–1105, 1981.
Boyde A, Maconnachie E, Reid SA, Delling G, Mundy GR: Scanning electron microscopy in bone pathology: Review of methods. Potential and applications. Scanning Electron Microscopy 4:1537–1554, 1986.
Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA, Bennett RC, Martin TJ: Localization of parathyroid hormone related protein in breast cancer metastases. Increased incidence in bone compared with other sites. Cancer Res 51:3059–3061, 1991.
Bouizar Z, Spyratos F, Deytieux S, Vernejeoul M-C, Jullienne A: Polymerase chain reaction analysis of parathyroid hormone related protein gene expression in breast cancer patients and occurrence of bone metastases. Cancer Res 53:5076–5078, 1993.
Arguello F, Baggs RB, Frantz CN: A murine model of experimental metastasis to bone and bone marrow. Cancer Res 48:6876–6881, 1988.
Nakai M, Mundy GR, Williams PJ, Boyce B, Yoneda T: A synthetic antagonist to laminin inhibits the formation of osteolytic metastases by human melanoma cells in nude mice. Cancer Res 52:5395–5399, 1992.
Fidler IJ: Rationale and methods for the use of nude mice to study the biology and therapy of human cancer metastasis. Cancer Metast Rev 5:29–49, 1986.
Crowley CW, Cohen RL, Lucas BK, Liu G, Shuman MA, Levison AD: Prevention of metastasis by inhibition of the urokinase receptor. Proc Natl Acad Sci USA 90:5021–5025, 1993.
Nordeen SK, Green PP, Fowlkes DM: A rapid, sensitive, and inexpensive assay for chloramphenicol acetyltransferase. DNA 6:173–178, 1987.
Fleisch H: Bisphosphonates: pharmacology and use in the treatment of tumor-induced hypercalcemic and metastatic bone disease. Drugs 42:919–944, 1991.
Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Gloub E, Rodan RA: Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 88:2095–2105, 1991.
Albelda SM, Buck CA: Integrins and other cell adhesion molecules. FASEB J 4:2868–2880, 1990.
Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, Takatsuka Y, Matsuyoohi N, Hirano S, Takeichi M, Mori T: Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res 53:1696–1701, 1993.
Mareel MM, Behrens J, Birchmeier W, De Bruyne GK, Vleminckx K, Hoogewijs A, Fiers WC, Van Roy FM: Down-regulation of E-cadherin expression in Madin-Darby canine kidney (MDCK) cells inside tumors of nude mice. Int J Cancer 47:922–928, 1991.
Haynes RO: Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11–25, 1992.
Seftor REB, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJC: Role of the αvβ3 integrin in human melanoma cell invasion. Proc Natl Acad Sci USA 89:1557–1561, 1992.
Takeichi M: Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251:1451–1454, 1991.
Takeichi M: Cadherins in cancer: Implications for invasion and metastasis. Curr Opin Cell Biol 5:806–811, 1993.
Behrens J, Mareel MM, Van Roy FM, Birchmeier W: Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol 108:2435–2447, 1989.
Vleminckx K, Vakaet L, Mareel M, Fiers W, Van Roy F: Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66:107–119, 1991.
Mahoney PA, Weber U, Onofrechuk P, Biessmann H, Bryant PJ, Goodman CS: Thefat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell 67:853–868, 1991.
Sommers CL, Thompson EW, Torri JA, Kemler R, Gelmann EP, Byers SW: Cell adhesion molecule uvomorulin expression in human breast cancer cell lines: Relationship to morphology and invasive capacities. Cell Growth Diff 2:365–371, 1991.
Kieran MW, Longenecker BM: Organ-specific metastasis with special reference to avian systems. Cancer Metast Rev 2:165–182, 1983.
Netland PA, Zetter BR: Organ-specific adhesion of metastatic tumor cellsin vitro. Science 224:1113–1115, 1984.
Haq M, Goltzman D, Tremblay G, Brodt P: Rat prostate adenocarcinoma cells disseminate to bone and adhere preferentially to bone marrow-derived endothelial cells. Cancer Res 52:4613–4619, 1992.
Nip J, Shibata H, Loskutoff DJ, Cheresh DA, Brodt P: Human melanoma cells derived from lymphatic mestastases use integrins αvβ3 to adhere to lymph node vitronectin. J Clin Invest 90:1406–1413, 1992.
Nicholson GL: Organ specificity of tumor metastasis: Role of preferential adhesion, invasion and growth of malignant cells at specific secondary sites. Cancer Metast Rev 7:143–188, 1988.
Globus RK, Plauet J, Gospodarowicz D: Cultured bovine bone cells synthesize basic fibroblast growth factor and store it in their extracellular matrix. Endocrinology 124:1539–1547, 1989.
Zucker S, Lysik RM, Zarrabi MH, Moll U: Mr 92,000 Type IV collagenase is increased in plasma of patients with colon cancer and breast cancer. Cancer Res 53:140–146, 1993.
De Clerck YA, Perez N, Shimada H, Boone TC, Langley KE, Taylor SM: Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Res 52:701–708, 1992.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yoneda, T., Sasaki, A. & Mundy, G.R. Osteolytic bone metastasis in breast cancer. Breast Cancer Res Tr 32, 73–84 (1994). https://doi.org/10.1007/BF00666208
Issue Date:
DOI: https://doi.org/10.1007/BF00666208