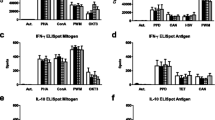
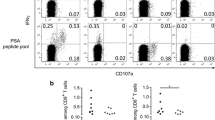
Summary
Several immunological in vitro tests were performed on peripheral blood mononuclear cells of patients with adenocarcinoma of the prostate, stages A, B, C, D. The cytotoxicity of effector natural killer cells towards K-562 targets decreased with increasing disease spread, while their percentage was not significantly changed. The proportion of CD4 (helper/inducer) cells tended to fall with tumor advance, but the proportion of CD8 (suppressor/cytotoxic) cells remained almost constant. Secretion of interleukin-2 from peripheral blood mononuclear cells was diminished with disease progression. Pretreatment of a patient's lymphocytes with cimetidine (antagonist of H-2-bearing suppressor T cells) or indomethacin (inhibitor of prostaglandin synthesis) enhanced natural killer activity.
Our data point to the existence of abberant immune functions in early stages of carcinoma of the prostate and to aggravation of these immune abnormalities in advanced disease.
Similar content being viewed by others
References
Allavena P, Introna M, Mangioni C, Mantovani A (1981) Inhibition of NK activity by tumor associated lymphoid cells from ascitic ovarian carcinomas. J Natl Cancer Inst 67:379
Balch CM, Dougherty PA, Tilden AB (1982) Executive prostaglandin E2 production by suppressor monocytes in head and neck cancer patients. Ann Surg 196:645
Bankhurst AD (1982) The modulation of human NK cell activity by prostaglandins. J Clin Lab Immunol 7:85
Barlozzari T, Reynolds CW, Herberman RB (1983) In vivo role of natural killer cells: involvement of large granular lymphocytes in the clearance of tumor cells in antiasialo GM1-treated rats. J Immunol 131:1024
Böyum A (1968) Separation of leucocytes from blood and bone marrow. Scand J Clin Invest 21 (suppl) 97:77
Braun DP, Harris JE (1981) Relationship of leucocyte numbers, immunoregulatory cell function and PHA responsiveness in cancer patients. J Natl Cancer Inst 67:809
Braun DP, Harris JE, Rubinstein M (1984) Relationship of arachidonic acid metabolism in indomethacin sensitive immunoregulatory function and lymphocyte PGE sensitivity in PBMC of disseminated solid tumor cancer patients. J Immunopharmacol 6:227
De Baer KP, Braun DP, Harris JE (1982) Natural cytotoxicity and antibody dependent cytotoxicity in solid tumor cancer patients. Regulation by adherent cells. Clin Immunol Immunopathol 23:133
Domzig W, Stadler BM, Herberman RB (1983) Interleukin-2 dependence of human NK cell activity. J Immunol 130:1970
Flodgren P, Sjogren O (1985) Influence in vitro on NK and K cell activities by cimetidine and indomethacin with and without simultaneous exposure to interferon. Cancer Immunol Immunother 19:28
Flodgren P, Bongstrom S, Jansson P, Lindstrom C, Sjogren HO (1983) Metastatic malignant melanoma: regression induced by combined treatment with interferon HUIFN-α (αe) and cimetidine. Int J Cancer 32:657
Gerson JM, Varesio L, Herberman RB (1981) Systemic and in situ NK activities in mice bearing progressively growing murine sarcoma virus induced tumors. Int J Cancer 27:243
Gillis S, Feanin M, Ou W, Smith KA (1980) T cell growth: parameters of production and quantitative microassay for activity. J Immunol 120:2027
Goodwin JS, Messner RP, Peake GT (1980) Prostaglandin suppression of mitogen stimulated dependent restoration of local graft versus host reaction among cells from cancer patients. J Natl Cancer Inst 65:317
Haller O, Hannson M, Kiessling R, Wigzell H (1977) Nonconventional NK cells may play a decisive role in providing resistance against syngeneic tumor cells in vivo. Nature 270:609
Han T, Takita H (1980) Indomethacin mediated enhancement of lymphocyte response to mitogens in healthy subjects and lung cancer patients. Cancer 46:2416
Harris JE, DeBaer KP, Vahey AL et al. (1982) The measurement of leucocyte subsets in the peripheral blood of cancer patients with solid tumors using monoclonal antibody regents. Med Pediat Oncol 10:185
Herberman RB, Munn ME, Lavnin DH (1975) Natural cytotoxicity activity of mouse lymphoid cells against syngeneic and allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer 16:216
Herr HW (1980) Suppressor cells in immunodepressed bladder and prostate cancer patients. J Urol 123:635
Herr HW (1981) Adherent suppressor cells in the blood of patients with bladder cancer. J Urol 126:457
Hinai U, Hill NO, Motoo Y, Osther K (1985) Cimetidine enhances interferon induced augmentation of NK cell activity and suppresses interferon production. Acta Pathol Microbiol Immunol Scand 93:153
Jewett NJ (1975) The present status of radical prostatectomy for stages A and B prostatic cancer. Urol Clin North Am 2:105
Karavodin LM, Giuliano AE, Golub SH (1981) T lymphocyte subsets in patients with malignant melanoma. Cancer Immunol Immunother 11:251
Kasai JC, Leclerc JC, McVay-Bodreau L, Shen FW, Cantor H (1979) Direct evidence that NK cells in non immune spleen cell populations prevent tumor growth in vivo. J Exp Med 149:1260
Lang NP, Ortaldo JR, Bonnard GP, Herberman RB (1982) Interferon and prostaglandins: effects on human natural and lectin induced cytotoxicity. J Natl Cancer Inst 69:339
Miyaska N, Darnell B, Baron S, Talal N (1984) Interleukin-2 enhances natural killing of normal lymphocytes. Cell Immunol 84:154
Ogden BE, Hill HR (1980) Histamine regulates lymphocyte mitogenic responses through activation of specific H1 and H2 histamine receptors. Immunology 41:107
Ortaldo JR, Herberman RB (1984) Heterogeneity of NK cells. Annu Rev Immunol 2:359
Osband ME, Hamilton D, Shen YJM, Cohen E, Shlesinger M, Lavin P, Brown A, MacCaffrey R (1981) Successful tumour immunotherapy with cimetidine in mice. Lancet i:636
Pollack SB, Hallenbeck LA (1982) In vivo reduction of NK activity with anti NK1 serum: direct evidence of NK cells in tumor clearance. Int J Cancer 29:203
Pross HF, Baines MG (1976) Spontaneous human lymphocyte mediated cytotoxicity against tumor target cells. I. The effect of malignant disease. Int J Cancer 18:593
Rocklin RE, Breand J, Gupta S, Good RA, Melman KL (1980) Characterization of the human blood lymphocytes that produce a histamine induced suppressor factor. Cell Immunol 51:226
Ruiz-Arguelles A, Serrogy HB, Ritts RE Jr (1982) In vitro effect of cimetidine on human cell mediated cytotoxicity. I. Inhibition of NK cell activity. Cell Immunol 69:1
Sahasnabudha DM, McCune CS, O'Donnell RW, Henshaw EC (1987) Inhibition of suppressor T lymphocytes by cimetidine. J Immunol 138:2760
Steinhauser EH, Doyle AT, Reed J, Kadish AN (1982) Defective natural cytotoxicity in patients with cancer: normal number of effector cells but decreased recycling capacity in patients with advanced disease. J Immunol 129:2256
Tutton PJM, Barkla DH (1983) Comparison of the tumor inhibitory effects of three histamine H-2 receptor antagonists. Cancer Res 3:7
Von Raenn J, Harris JE, Braun DP (1987) Suppressor cell function in solid tumor cancer patients. J Clin Oncol 5:150
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lahat, N., Alexander, B., Dan Levin, R. et al. The relationship between clinical stage, natural killer activity and related immunological parameters in adenocarcinoma of the prostate. Cancer Immunol Immunother 28, 208–212 (1989). https://doi.org/10.1007/BF00204990
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00204990